Canada Gazette, Part I, Volume 158, Number 22: Regulations Amending Certain Regulations Concerning Controlled Substances
June 1, 2024
Statutory authorities
Financial Administration Act
Food and Drugs Act
Controlled Drugs and Substances Act
Sponsoring department
Department of Health
REGULATORY IMPACT ANALYSIS STATEMENT
For the Regulatory Impact Analysis Statement, see the Controlled Substances Regulations.
PROPOSED REGULATORY TEXT
Notice is given that the Governor in Council proposes to make the annexed Regulations Amending Certain Regulations Concerning Controlled Substances under
- (a) paragraph 19.1(a)footnote a of the Financial Administration Act footnote b;
- (b) section 30footnote c of the Food and Drugs Act footnote d; and
- (c) subsection 55(1)footnote e of the Controlled Drugs and Substances Act footnote f.
Interested persons may make representations concerning the proposed Regulations within 60 days after the date of publication of this notice. They are strongly encouraged to use the online commenting feature that is available on the Canada Gazette website but if they use email, mail or any other means, the representations should cite the Canada Gazette, Part I, and the date of publication of this notice, and be sent to the Office of Legislative and Regulatory Affairs, Controlled Substances Directorate, Controlled Substances and Cannabis Branch, Department of Health, Address Locator: 0302A, 150 Tunney’s Pasture Driveway, Ottawa, Ontario K1A 0K9 (email: csd.regulatory.policy-politique.reglementaire.dsc@hc-sc.gc.ca).
Ottawa, May 23, 2024
Wendy Nixon
Assistant Clerk of the Privy Council
Regulations Amending Certain Regulations Concerning Controlled Substances
Financial Administration Act
Licensed Dealers for Controlled Drugs and Narcotics (Veterinary Use) Fees Regulations
1 The definitions controlled drug, dealer’s licence and narcotic in section 1 of the Licensed Dealers for Controlled Drugs and Narcotics (Veterinary Use) Fees Regulations footnote 1 are replaced by the following:
- controlled drug
- has the same meaning as in subsection 1(1) of the Controlled Substances Regulations. (drogue contrôlée)
- dealer’s licence
- means a licence that is issued under subsection 12(1) of the Controlled Substances Regulations and that relates to a narcotic or controlled drug. (licence de distributeur autorisé)
- narcotic
- has the same meaning as in subsection 1(1) of the Controlled Substances Regulations. (stupéfiant)
Fees in Respect of Dealer’s Licences Regulations
2 Subsection 2(1) of the Fees in Respect of Dealer’s Licences Regulations footnote 2 is replaced by the following:
Purpose — fees
2 (1) The purpose of these Regulations is to prescribe the fees for the examination of an application for a dealer’s licence under the Controlled Substances Regulations that relates to a narcotic or controlled drug, as defined in subsection 1(1) of those Regulations, or for the examination of an application for the renewal of such a licence.
3 Section 29 of the Regulations is replaced by the following:
Definitions
29 (1) The following definitions apply in sections 30, 31 and 33.
- dealer’s licence
- means a licence that is issued under subsection 12(1) of the Controlled Substances Regulations and that relates to a narcotic or controlled drug, as defined in subsection 1(1) of those Regulations. (licence de distributeur autorisé)
- health care facility
- means a facility that provides diagnostic or therapeutic services to patients. It includes a group of such facilities that report to one common management that has responsibility for the activities carried out in those facilities. (établissement de santé)
Words and expressions
(2) Unless the context otherwise requires, all other words and expressions used in sections 30, 31 and 33 have the meaning assigned to them by the Controlled Drugs and Substances Act or the Controlled Substances Regulations.
4 Subsection 30(2) of the Regulations is replaced by the following:
Non-application — narcotic or controlled drug for veterinary use only
(2) These Regulations do not apply to a narcotic or controlled drug that is for veterinary use only.
5 Subsection 31(3) of the Regulations is replaced by the following:
Timing of payment
(3) Subject to subsection (4), the fee is payable at the time that the application for a dealer’s licence or for the renewal of a dealer’s licence is submitted under section 11 or 15 of the Controlled Substances Regulations.
Food and Drugs Act
Food and Drug Regulations
6 The definition nurse practitioner in subsection B.25.019(2) of the Food and Drug Regulations footnote 3 is replaced by the following:
- nurse practitioner
- has the same meaning as in subsection 1(1) of the Controlled Substances Regulations. (infirmier praticien)
7 Subparagraphs C.01.004(1)(b)(i) to (iv) of the Regulations are replaced by the following:
- (i) in the case of a prescription drug, the symbol “Pr”, which must not appear on the label of any other drug,
- (ii) in the case of a drug that is a narcotic, as defined in subsection 1(1) of the Controlled Substances Regulations, the symbol “N” in a colour contrasting with the rest of the label or in type not less than half the size of any other letter used on the label,
- (iii) in the case of a drug that is a controlled drug, as defined in subsection 1(1) of the Controlled Substances Regulations, other than one contained in a finished product referred to in section 3 of those Regulations, the following symbol in a clear manner and a conspicuous colour and size, and
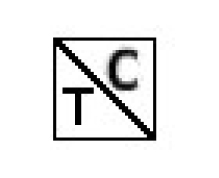
- (iv) in the case of a drug that is a targeted substance, as defined in subsection 1(1) of the Controlled Substances Regulations, the following symbol in a colour contrasting with the rest of the label and in type not less than half the size of any other letter used on the main panel,
8 Paragraph C.01.028(2)(c) of the Regulations is replaced by the following:
- (c) a prescription drug or a drug that is a narcotic, controlled drug or targeted substance, as defined in subsection 1(1) of the Controlled Substances Regulations, and that is required to be sold under a prescription in accordance with those Regulations.
9 (1) The portion of subsection C.01.031.2(1) of the French version of the Regulations before paragraph (a) is replaced by the following:
C.01.031.2 (1) Les articles C.01.029 à C.01.031 ne s’appliquent pas aux drogues suivantes :
(2) Paragraph C.01.031.2(1)(a) of the Regulations is replaced by the following:
- (a) a prescription drug or a drug that is a narcotic, controlled drug or targeted substance, as defined in subsection 1(1) of the Controlled Substances Regulations, and that is required to be sold under a prescription in accordance with those Regulations;
10 Paragraphs C.01.048(1)(a) to (d) of the Regulations are replaced by the following:
- (a) a drug that is a narcotic or controlled drug, as defined in subsection 1(1) of the Controlled Substances Regulations; or
- (b) a prescription drug, as defined in subsection 1(2) of the Cannabis Regulations.
11 Paragraph C.01.050(4)(b) of the Regulations is replaced by the following:
- (b) drugs that are narcotics, controlled drugs or targeted substances, as defined in subsection 1(1) of the Controlled Substances Regulations, and that are required to be sold under a prescription in accordance with those Regulations; and
12 Paragraph C.01.061(2)(c) of the Regulations is replaced by the following:
- (c) where the drug is a narcotic or controlled drug, as defined in subsection 1(1) of the Controlled Substances Regulations, no package contains more than the number of dosage units shown on the label except as provided in the table to this section.
13 (1) Paragraph (a) of the definition wholesaler in subsection C.01A.001(1) of the Regulations is replaced by the following:
- (a) a drug in dosage form that is listed in Schedule C or D to the Act, a drug that is a prescription drug;
(2) Paragraph (c) of the definition wholesaler in subsection C.01A.001(1) of the Regulations is replaced by the following:
- (c) a drug that is a narcotic or controlled drug, as defined in subsection 1(1) of the Controlled Substances Regulations; or
14 Paragraphs C.01A.004(3)(a) to (c) of the Regulations are replaced by the following:
- (a) in the case of an activity with respect to a drug that is a narcotic or controlled drug, as defined in subsection 1(1) of the Controlled Substances Regulations, a dealer’s licence issued under subsection 12(1) of those Regulations to conduct that activity in accordance with those Regulations;
- (b) in the case of an activity with respect to a drug containing cannabis, as defined in subsection 2(1) of the Cannabis Act, a licence issued under the Cannabis Regulations to conduct that activity in accordance with those Regulations.
15 Subparagraph C.01A.005(1)(j)(i) of the Regulations is replaced by the following:
- (i) for each drug for which the licence is requested that is a narcotic or controlled drug, as defined in subsection 1(1) of the Controlled Substance Regulations, or a drug containing cannabis, as defined in subsection 2(1) of the Cannabis Act, and
| Item | Categories of drugs |
|---|---|
| 6 | Prescription drugs or drugs that are narcotics or controlled drugs, as defined in subsection 1(1) of the Controlled Substances Regulations, and drugs containing cannabis, as defined in subsection 2(1) of the Cannabis Act |
17 Section C.09.001 of the Regulations is replaced by the following:
C.09.001 This Division does not apply to
- (a) a drug that is required by these Regulations to be sold under a prescription;
- (b) a drug that is a narcotic, as defined in subsection 1(1) of the Controlled Substances Regulations, and that is required under those Regulations to be sold under a prescription; or
- (c) a drug intended for use exclusively in animals.
Medical Devices Regulations
18 Subsection 3(2) of the Medical Devices Regulations footnote 4 is replaced by the following:
(2) Subsection (1) does not apply to the following drugs:
- (a) a drug listed in Schedule E or F to the Act;
- (b) a drug listed in the Schedules to the Controlled Substances Regulations;
- (c) a drug, as defined in subsection 1(2) of the Cannabis Regulations, containing cannabis, as defined in subsection 2(1) of the Cannabis Act.
Controlled Drugs and Substances Act
Precursor Control Regulations
19 Paragraphs 6.1(a) and (b) of the Precursor Control Regulations footnote 5 are replaced by the following:
- (a) a dealer’s licence issued under subsection 12(1) of the Controlled Substances Regulations for the production of the controlled substance within the meaning of those Regulations; or
- (b) an exemption issued under subsection 56(1) of the Act.
20 (1) Paragraph 14(4)(b) of the Regulations is replaced by the following:
- (b) a document issued by a Canadian police force or a company that is accredited by the Royal Canadian Mounted Police with respect to each of the persons referred to in paragraph (a), setting out the person’s criminal record for the previous 10 years, as an adult, in respect of designated drug offences and designated criminal offences, or indicating that the person has no such record;
(2) Subsection 14(5) of the Regulations is repealed.
21 Paragraph 60(3)(b) of the Regulations is replaced by the following:
- (b) a document issued by a Canadian police force or a company that is accredited by the Royal Canadian Mounted Police, setting out the senior person in charge’s criminal record for the previous 10 years, as an adult, in respect of the offences mentioned in paragraph (a), or indicating that the person has no such record; and
Coming into Force
22 These Regulations come into force on the 365th day after the day on which they are published in the Canada Gazette, Part II.
Terms of use and Privacy notice
Terms of use
It is your responsibility to ensure that the comments you provide do not:
- contain personal information
- contain protected or classified information of the Government of Canada
- express or incite discrimination on the basis of race, sex, religion, sexual orientation or against any other group protected under the Canadian Human Rights Act or the Canadian Charter of Rights and Freedoms
- contain hateful, defamatory, or obscene language
- contain threatening, violent, intimidating or harassing language
- contain language contrary to any federal, provincial or territorial laws of Canada
- constitute impersonation, advertising or spam
- encourage or incite any criminal activity
- contain external links
- contain a language other than English or French
- otherwise violate this notice
The federal institution managing the proposed regulatory change retains the right to review and remove personal information, hate speech, or other information deemed inappropriate for public posting as listed above.
Confidential Business Information should only be posted in the specific Confidential Business Information text box. In general, Confidential Business Information includes information that (i) is not publicly available, (ii) is treated in a confidential manner by the person to whose business the information relates, and (iii) has actual or potential economic value to the person or their competitors because it is not publicly available and whose disclosure would result in financial loss to the person or a material gain to their competitors. Comments that you provide in the Confidential Business Information section that satisfy this description will not be made publicly available. The federal institution managing the proposed regulatory change retains the right to post the comment publicly if it is not deemed to be Confidential Business Information.
Your comments will be posted on the Canada Gazette website for public review. However, you have the right to submit your comments anonymously. If you choose to remain anonymous, your comments will be made public and attributed to an anonymous individual. No other information about you will be made publicly available.
Comments will remain posted on the Canada Gazette website for at least 10 years.
Please note that public email is not secure, if the attachment you wish to send contains sensitive information, please contact the departmental email to discuss ways in which you can transmit sensitive information.
Privacy notice
The information you provide is collected under the authority of the Financial Administration Act, the Department of Public Works and Government Services Act, the Canada–United States–Mexico Agreement Implementation Act,and applicable regulators’ enabling statutes for the purpose of collecting comments related to the proposed regulatory changes. Your comments and documents are collected for the purpose of increasing transparency in the regulatory process and making Government more accessible to Canadians.
Personal information submitted is collected, used, disclosed, retained, and protected from unauthorized persons and/or agencies pursuant to the provisions of the Privacy Act and the Privacy Regulations. Individual names that are submitted will not be posted online but will be kept for contact if needed. The names of organizations that submit comments will be posted online.
Submitted information, including personal information, will be accessible to Public Services and Procurement Canada, who is responsible for the Canada Gazette webpage, and the federal institution managing the proposed regulatory change.
You have the right of access to and correction of your personal information. To seek access or correction of your personal information, contact the Access to Information and Privacy (ATIP) Office of the federal institution managing the proposed regulatory change.
You have the right to file a complaint to the Privacy Commission of Canada regarding any federal institution’s handling of your personal information.
The personal information provided is included in Personal Information Bank PSU 938 Outreach Activities. Individuals requesting access to their personal information under the Privacy Act should submit their request to the appropriate regulator with sufficient information for that federal institution to retrieve their personal information. For individuals who choose to submit comments anonymously, requests for their information may not be reasonably retrievable by the government institution.