Nicotine Concentration in Vaping Products Regulations: SOR/2021-123
Canada Gazette, Part II, Volume 155, Number 13
Registration
SOR/2021-123 June 10, 2021
TOBACCO AND VAPING PRODUCTS ACT
CANADA CONSUMER PRODUCT SAFETY ACT
P.C. 2021-518 June 10, 2021
His Excellency the Administrator of the Government of Canada in Council, on the recommendation of the Minister of Health, makes the annexed Nicotine Concentration in Vaping Products Regulations pursuant to
- (a) sections 7.8footnote a, 17footnote b and 33footnote c of the Tobacco and Vaping Products Actfootnote d; and
- (b) section 37footnote e of the Canada Consumer Product Safety Actfootnote f.
Nicotine Concentration in Vaping Products Regulations
Definitions
Definitions
1 The following definitions apply in these Regulations.
- Act
- means the Tobacco and Vaping Products Act. (Loi)
- vaping substance
- has the meaning assigned by paragraph (d) of the definition vaping product in section 2 of the Act. (substance de vapotage)
Application
Retail sale — vaping products
2 (1) These Regulations apply to every vaping product that is intended for retail sale in Canada, as well as to its packaging.
Vaping products — otherwise furnished
(2) These Regulations also apply to every vaping product that is intended to be otherwise furnished, in Canada, at a point of sale that is a retail establishment where vaping products are ordinarily sold, as well as to the packaging of such a vaping product.
Non-application
3 These Regulations do not apply to a vaping product that is the subject of an authorization, including a licence, issued under the Food and Drugs Act authorizing its sale.
Nicotine Concentration
Standard — maximum nicotine concentration
4 (1) For the purposes of section 7.2 of the Act, a vaping product must not contain nicotine in a concentration that exceeds 20 mg/mL when the vaping substance is tested using the International Organization for Standardization standard ISO 20714, entitled E-liquid — Determination of nicotine, propylene glycol and glycerol in liquids used in electronic nicotine delivery devices — Gas chromatographic method, as amended from time to time.
Conversion of units of measure
(2) The nicotine concentration expressed in mg/mL must be obtained by multiplying the results of the test conducted in accordance with ISO 20714, expressed in mg/g, by the density of the vaping substance, expressed in g/mL.
Interpretation — vaping substance
(3) For the purposes of these Regulations, a reference to “e-liquid” in ISO 20714 must be read as a reference to “vaping substance”.
Prohibitions — packaging and sale
5 For the purposes of section 30.45 of the Act, a vaping product must not be packaged or sold in a package that displays a nicotine concentration statement, referred to in section 5 of the Vaping Products Labelling and Packaging Regulations, that indicates that the nicotine concentration in the vaping substance exceeds 20 mg/mL.
Transitional Provision, Consequential Amendments and Coming into Force
Transitional Provision
Retailers
6 Despite these Regulations, a retailer may, until the day that is the 15th day after the day on which these Regulations come into force, sell a vaping product that is packaged in a manner that is contrary to section 5.
Consequential Amendments to the Vaping Products Labelling and Packaging Regulations
7 Subsection 2(2) of the Vaping Products Labelling and Packaging Regulationsfootnote 1 is replaced by the following:
Vaping products — otherwise furnished
(2) This Part also applies to every vaping product that is intended to be otherwise furnished, in Canada, at a point of sale that is a retail establishment where vaping products are ordinarily sold, as well as to the packaging of such a vaping product.
8 Section 49 of the Regulations is replaced by the following:
Maximum nicotine concentration
49 Subject to the Nicotine Concentration in Vaping Products Regulations, a vaping product must not contain nicotine in a concentration of 66 mg/mL or more.
Coming into Force
15th day after publication
9 These Regulations come into force on the 15th day after the day on which they are published in the Canada Gazette, Part II.
REGULATORY IMPACT ANALYSIS STATEMENT
(This statement is not part of the Regulations.)
Executive summary
Issues: There has been a rapid increase in youth vaping in Canada. Young persons are being exposed to vaping product-related harms, including those related to nicotine exposure, which can result in a dependence on nicotine and an increased risk of tobacco use. Health Canada has identified the availability of high-nicotine-concentration vaping products in the Canadian market since 2018 as one of the key factors that have contributed to the rapid rise in youth vaping.
Description: The Nicotine Concentration in Vaping Products Regulations (the Regulations) establish a maximum nicotine concentration of 20 mg/mL for vaping products manufactured or imported for sale in Canada and prohibit the packaging and sale of vaping products if the nicotine concentration displayed on the package exceeds that value. The Regulations also amend the Vaping Products Labelling and Packaging Regulations (VPLPR) to align with this limit for products intended for the domestic market, while continuing to prohibit a nicotine concentration of 66 mg/mL or more in vaping products intended for export.
Rationale: Lowering the maximum concentration of nicotine allowed in vaping products is expected to contribute to reducing the appeal of these products to youth, which would help address the rapid rise in youth vaping.
The Regulations will support Canada's Tobacco Strategy (CTS), which aims to reduce the burden of disease and death from tobacco use and its consequential impact on the health care system and society. The Regulations are expected to primarily benefit youth by contributing to the reduction in the number of those experimenting with vaping products, who could otherwise be exposed to and dependent on nicotine and transition into tobacco users. There will be long-term benefits in terms of avoided tobacco-related mortality and morbidity, including from exposure to second-hand smoke.
The Regulations will result in total incremental costs for the vaping industry estimated at $452.0 million present value (PV) over 30 years (or about $36.4 million in annualized value). The monetized costs to the vaping industry are associated with the disposal of their stocks of vaping products above 20 mg/mL nicotine, as these will no longer be sold or distributed, and potential industry profit losses. Implementation of the Regulations will result in a one-time cost of $18,682 PV over 30 years (or about $1,506 in annualized value) to Health Canada to produce compliance promotion material. There will be no incremental costs to Health Canada from performing compliance and enforcement activities.
A break-even analysis indicates that a decrease in the vaping initiation rates in the range of 2.58% to 4.11% relative to the baseline initiation rate would be sufficient to produce public health benefits equivalent to or greater than the estimated monetized costs.
The small business lens applies. There is no administrative burden on businesses that would result from the Regulations; therefore, the one-for-one rule does not apply.
The Regulations align with restrictions in place in the provinces of British Columbia and Nova Scotia. They also align with measures in place in the European Union, Iceland, Israel, Moldova, Saudi Arabia and the United Kingdom. They do not align with measures in the United States, as there is currently no restriction on the nicotine concentration of vaping products at the federal level.
Issues
There has been a rapid increase in youth vaping in Canada. Data from the 2018–2019 Canadian Student Tobacco, Alcohol and Drugs Survey (CSTADS) indicates that the prevalence of vaping has doubled among students compared to the previous survey in 2016–2017. Young persons are being exposed to vaping product-related harms, including those related to nicotine exposure, which can result in a dependence on nicotine and an increased risk of tobacco use. Health Canada is also concerned that the use of vaping products could renormalize smoking behaviour.
The introduction of high-nicotine-concentration vaping products to the Canadian market in 2018 is believed to have contributed to the rapid rise in youth vaping.
Background
In response to the 2015 report of the House of Commons' Standing Committee on Health entitled Vaping: Toward a Regulatory Framework for E-Cigarettes, Parliament established a new legislative framework. An Act to amend the Tobacco Act and the Non-smokers' Health Act and to make consequential amendments to other Acts received royal assent on May 23, 2018. As a consequence, vaping products are subject to the Tobacco and Vaping Products Act (TVPA) and either the Food and Drugs Act (FDA) or the Canada Consumer Product Safety Act (CCPSA), depending on whether or not the product is marketed for therapeutic use. The provisions of the TVPA apply to all vaping products, including those regulated under the FDA, except where they are expressly excluded from the application of the TVPA and some of its provisions (e.g. through the Regulations Excluding Certain Vaping Products Regulated Under the Food and Drugs Act from the Application of the Tobacco and Vaping Products Act).
The overall objective of the TVPA with respect to vaping products is to prevent vaping product use from leading to the use of tobacco products by young persons and non-users of tobacco products. Specifically, it aims to
- (1) protect young persons and non-users of tobacco products from inducements to use vaping products;
- (2) protect the health of young persons and non-users of tobacco products from exposure to and dependence on nicotine that could result from the use of vaping products;
- (3) protect the health of young persons by restricting access to vaping products;
- (4) prevent the public from being deceived or misled with respect to the health hazards of using vaping products; and
- (5) enhance public awareness of those hazards.
To this end, the TVPA regulates, in addition to tobacco, the manufacture, sale, labelling and promotion of vaping products. Several provincial and territorial jurisdictions have also adopted measures to regulate vaping products to varying degrees and through different approaches (see section “Regulatory cooperation and alignment” for further details).
Canada's Tobacco Strategy
Tobacco use is the leading preventable cause of disease and premature death in Canada. It is a known or probable cause of more than 40 debilitating and often fatal diseases of the lungs, heart, and other organs, and is responsible for over 47 000 premature deaths every year in Canada. Tobacco products contain nicotine, a highly addictive substance that is responsible for tobacco dependence and consequent repeated long-term use that results in chronic exposure to harmful chemicals. Health and economic costs associated with tobacco use in Canada were estimated at $12.3 billion for the year 2017.footnote 2
Canada's Tobacco Strategy (CTS), introduced in 2018, features broad, population-based approaches to achieve the ambitious target of less than 5% tobacco use prevalence by 2035, with targeted approaches focused on specific populations suffering from high levels of tobacco use. One of the CTS objectives is to protect youth and non-tobacco users from nicotine addiction.
For persons who smoke, the best thing they can do to improve their health is to quit smoking. However, the CTS notes that giving adults who smoke access to less harmful options than cigarettes will help reduce their health risks and possibly save lives. There is a growing body of evidence indicating that vaping products, while not harmless, are a source of nicotine that is a less harmful alternative to smoking if a person who smokes switches completely to vaping products; this can reduce one's exposure to the many toxic and/or cancer-causing chemicals from smoking tobacco.
Health concerns and nicotine addiction
Vaping products are harmful. They emit an aerosol that contains potentially harmful chemicals. The inhalation of these chemicals into the lungs may have a negative impact on health, especially for youth and non-users of tobacco products. Most vaping products contain nicotine. Children and youth are especially susceptible to the harmful effects of nicotine, including addiction. Youth can become dependent on nicotine at lower levels of exposure than adults do.footnote 3 Exposure to nicotine during adolescence can also negatively alter brain development, including long-term effects on memory and concentration abilities.footnote 4,footnote 5,footnote 6
The report entitled Public Health Consequences of E-Cigarettes, published in 2018 by the U.S. National Academies of Sciences, Engineering, and Medicine (NASEM), represents expert consensus resulting from an independent, systematic review of a high volume of peer-reviewed scientific studies.footnote 7The report offers three conclusions that are of particular significance in supporting the need to further protect youth and non-users of tobacco products:
- (1) there is substantial evidence that the use of an e-cigarette results in symptoms of dependence;
- (2) there is conclusive evidence that in addition to nicotine, most e-cigarette products contain and emit numerous potentially toxic substances; and
- (3) there is substantial evidence that e-cigarette use increases the risk of ever using combustible tobacco cigarettes among youth and young adults.
Youth vaping
Data from the 2018–2019 CSTADS indicates that the prevalence (past 30 days) of vaping had doubled among students compared to the previous survey in 2016–2017.footnote 8 Twenty percent of students (418 000 individuals) in grades 7 to 12 (Secondary I through V in Quebec) had used an e-cigarette in the past 30 days, double the 10% from 2016–2017. In 2018–2019, the past-30-day prevalence was 11% (115 000) among students in grades 7 to 9 (Secondary I to III in Quebec) and 29% (304 000) among students in grades 10 to 12 (Secondary IV and V in Quebec).footnote 9Further data is presented in Figure 1. It was found that frequency of use is high, particularly in the upper grades: the prevalence of daily or almost daily e-cigarette use was 13% (133 000) among students in grades 10 to 12. As a comparison, the prevalence of daily cigarette use among students in grades 10 to 12 was 1% (14 000) in 2018–2019 (Figure 2).
Figure 1: Past-30-day e-cigarette use grouped by grade (CSTADS)
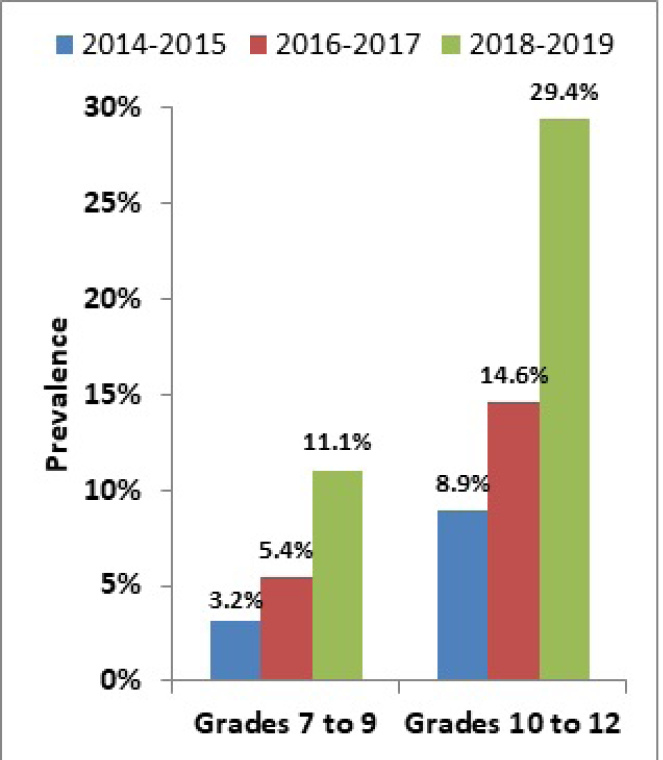
Figure 1 - Text version
| Year | Prevalence of past-30-day use of e-cigarettes by students in grades 7 to 9 (CSTADS) |
|---|---|
| 2014-2015 | 3.2 % |
| 2016-2017 | 5.4 % |
| 2018-2019 | 11.1 % |
| Year | Prevalence of past-30-day use of e-cigarettes by students in grades 10 to 12 (CSTADS) |
|---|---|
| 2014-2015 | 8.9 % |
| 2016-2017 | 14.6 % |
| 2018-2019 | 29.4 % |
Figure 2: Daily cigarette smoking and daily / almost daily e-cigarette use, grades 10 to 12 (2018–2019 CSTADS)
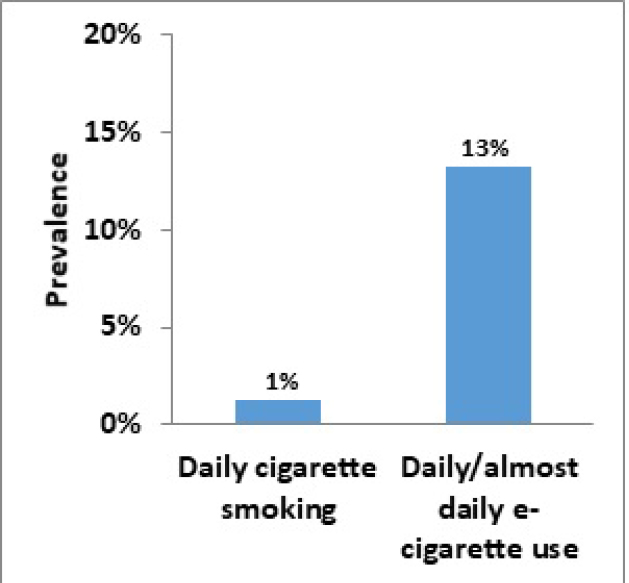
Figure 2 - Text version
| By students in grades 10-12 (2018–2019 CSTADS) | Prevalence |
|---|---|
| Daily cigarette smoking | 1 % |
| Daily/almost daily e-cigarette use | 13 % |
The vaping rate among youth (15 to 19 years old) remains high. Data from the 2019 and 2020 Canadian Tobacco and Nicotine Survey (CTNS) suggests that the rapid increase in youth vaping, observed between 2017 and 2019, may be levelling off, as there was no statistical difference between the 2019 and 2020 past-30-day prevalence rate (15% vs. 14%).footnote 10 It remains to be seen whether this survey results, while being encouraging, will translate into a downward trend in the prevalence of youth vaping in the future. This would only be possible by collecting and analyzing survey results over several cycles.
Adults who use vaping products
Data from the 2020 CTNS shows that the prevalence of past-30-day vaping was 13% among young adults aged 20 to 24, and 3% among adults aged 25 years and over. Young adults aged 20 to 24 reported that the three most common reasons for vaping were because they enjoyed it (24%), to reduce stress (24%) and to reduce or quit smoking (22%). Among adults aged 25 years and over, the top reason was to reduce or quit smoking (53%). Among those 25 years of age and older who vape, 46% had also smoked daily or occasionally in the past 30 days (current smokers). The picture is slightly different among adults aged 20 to 24 who vape, where 29% were current smokers.
Addressing the rise in youth vaping
Health Canada is taking action to address the rise of youth vaping. The Department is concerned that young persons are being exposed to vaping product-related harms, including those related to nicotine exposure, which can result in a dependence on nicotine and an increased risk of tobacco use and adverse health effects. Canada's public health achievements in tobacco control are at risk of being eroded if young persons who experiment with vaping products develop a dependence on nicotine, particularly those who would not otherwise have tried smoking.
Health Canada has invested more than $13 million in a national, targeted youth-oriented public information campaign, Consider the Consequences, to increase youth awareness of the harms of vaping. The campaign, launched in early 2019, comprises several components:
- Advertising: It is estimated that digital advertisements had been seen more than 840 million times as of October 2020. An evaluation of the advertising campaign found that 26% of teens who reported having seen the advertisements decided not to vape as a result.
- Experiential events: As of December 2020, experiential event tours in schools and community venues across the country had engaged 90 683 students in person and 1 694 students virtually.
- Distribution of resources: Since January 2019, over 40 000 online and print resources have been distributed to youth, parents and educators. From April 2019 to March 2021, over 595 000 visits to the campaign page have been registered.
- Influencers: In 2019–2020, digital influencers who targeted parents and youth were very effective in amplifying campaign messages through a variety of social media content.
Health Canada is working with other levels of governments, the medical community and other stakeholders to address multi-jurisdictional issues and enhance national cooperative and collaborative efforts to protect young persons and non-users of tobacco products from the health hazards of vaping. Grants of $12.4 million have been allocated over six years to address tobacco use and youth vaping through the Substance Use and Addictions Program. In particular, non-governmental and academic partners are using the funds to focus on youth vaping cessation projects or projects that have youth vaping cessation components. For example, a project with the University of Toronto ($1.3 million) will significantly enhance existing app-based approaches to support vaping cessation among youth and young adults, while another one is allowing the University of Waterloo ($1.1 million) to expand its survey capacity — including the use of biomarkers to better understand youth vaping behaviour patterns and measure their risk exposure.
Health Canada has taken action to help address the rise in youth vaping through the Vaping Products Promotion Regulations (VPPR) and the Vaping Products Labelling and Packaging Regulations (VPLPR).
The VPPR, made in June 2020, set out measures to further restrict the promotion of vaping products to youth. Subject to limited exceptions, the VPPR prohibit the promotion of vaping products and vaping product-related brand elements by means of advertising done in a manner that allows the advertising to be seen or heard by young persons. They also prohibit the display of vaping products and vaping product-related brand elements at points of sale, which allows the product or brand elements to be seen by young persons. This includes online points of sale. These measures help protect youth from inducements to using vaping products.
The VPPR also require that vaping product advertising conveys a warning about the health hazards of using vaping products. This is subject to certain exceptions, including for advertising at a point of sale that indicates only the availability and price of vaping products. The VPPR also set out the conditions for the presentation of the health warning and of the attribution to Health Canada for both audio and visual vaping advertisements. The objective of the health warnings on permitted advertising is to enhance public awareness about the health hazards of using vaping products.
The VPLPR, made in December 2019, establish two sets of requirements: Part 1 sets out labelling requirements pursuant to the TVPA, while Part 2 sets out labelling requirements and child-resistant container requirements pursuant to the CCPSA.
Part 1 of the VPLPR requires the display of two labelling elements for vaping products that contain nicotine: a nicotine concentration statement and a health warning about the addictiveness of nicotine. These labelling elements must be displayed on the vaping products and/or their packaging. Part 1 also sets out three permitted expressions that may be used on the product or its packaging when a vaping substance does not contain nicotine and, in the case of any other vaping product that contains a vaping substance, when the product is without nicotine. The specific objectives of this part are to enhance the awareness of the health hazards of using vaping products and to prevent the public from being deceived or misled with respect to the health hazards posed by their use.
Part 2 of the VPLPR
- requires a list of ingredients for all vaping substances on product labels;
- prohibits vaping products with nicotine concentrations of 66 mg/mL or more;
- requires information to warn about the toxicity of nicotine when ingested (including a first aid treatment statement); and
- requires refillable vaping products, including devices and their parts that contain nicotine, to be child-resistant.
The objective of this part is to protect the health and safety of young children by reducing the risk that they ingest vaping substances containing toxic concentrations of nicotine. The nicotine concentration limit was set at 66 mg/mL or more to address the risks associated with acute poisoning if nicotine in these products is ingested.
Appeal of high-nicotine-concentration vaping products
In 2016, the domestic vaping substances (e-liquid) market was valued at approximately $250 million. Vaping products that contained a low nicotine concentration (3 to 6 mg/mL) made up 60% ($150 million) of this market, while nicotine-free vaping products made up 18% ($45 million). Vaping products with a nicotine concentration that exceeded 18 mg/mL made up less than 10% of the market (less than $25 million).footnote 11 Therefore, prior to 2018, the vaping substances market in Canada was composed almost entirely of products below 20 mg/mL nicotine.
In 2018, a new generation of vaping products were introduced to the Canadian market, characterized by high concentrations of nicotine in salt form (called “nicotine salts”) that made nicotine less aversive when inhaled.footnote 12 As a result, vaping products above 20 mg/mL nicotine (a majority of which contained nicotine salts) quickly took a dominant market position, capturing 62% of the domestic market by value of nicotine-containing vaping substances in 2019.footnote 13 footnote 14
Information collected from 738 respondents for the 2019 Wave 3 International Tobacco Control Youth Tobacco and Vaping Survey indicates that, of youth in Canada aged 16 to 19 who had vaped in the past 30 days, 24% reported that they use vaping products “for the nicotine” among their top five reasons. The other four reasons included “for fun/I like it” (50%), “for the flavour” (40%), “curiosity/to try something new” (39%) and “to deal with stress or anxiety” (35%).footnote 15
Research using focus groups made up of 103 youth aged 13 to 19, conducted for Health Canada in March 2020, underscores the importance many place on vaping products with higher concentrations of nicotine. In this study, youth who reported using vaping products containing at least 50 mg/mL mentioned experiencing a “head rush” or “buzz” as by far “the best part about vaping.” Vaping makes most youth feel “relaxed,” but some also believe it makes them feel high and lightheaded. Other youth say vaping makes them feel lethargic, while for another group, vaping seems to energize them.footnote 16
In a recent survey of Canadians aged 15 and older conducted for Health Canada, a total of 1 232 respondents who vape were asked to identify the nicotine strength used on the basis of either percentage (%) or weight by volume (mg/mL) as per information displayed on their product.footnote 17 The survey findings point to differences by age in nicotine concentrations used. The majority (51%) of respondents reported using vaping products with less than 20 mg/mL or 2% nicotine, 36% reported using products with nicotine concentration equal to or above 20 mg/mL or 2%, and 13% indicated not knowing the nicotine concentration. Older adults (25 years and over) were more likely (54%) to report using vaping products with less than 20 mg/mL or 2% nicotine, as compared to youth vapers (15 to 19 years) [42%]. Use of vaping products with less than 20 mg/mL or 2% nicotine among young adults (aged 20 to 24 years) was not significantly different from the other age groups (47%). Youth vapers were more likely (45%) to report using vaping products with nicotine concentration equal to or above 20 mg/mL or 2%, as compared to older adults (33%). Use of vaping products with nicotine concentration equal to or above 20 mg/mL or 2% among young adults was not significantly different from the other age groups (38%).footnote 18
The availability of high-nicotine-concentration vaping products in the Canadian market since 2018 is not the only factor believed to have contributed to the rise in youth vaping. Other key factors include an increase in promotional activities relating to vaping products, including on social media, and the use of a wide variety of flavours and innovative design features. In addition to the Nicotine Concentration in Vaping Products Regulations, Health Canada has implemented regulations to address promotional activities, and is considering additional measures to address the other factors contributing to the rise in youth vaping, such as restricting flavours in vaping products.
Objective
The objective of the Nicotine Concentration in Vaping Products Regulations (the Regulations) is to protect young persons from inducements to use vaping products by lowering the concentration of nicotine to a maximum of 20 mg/mL. This is expected to contribute to reducing their appeal to youth.
The Regulations, in association with other vaping-related measures under the TVPA, aim to prevent vaping product use from leading to the use of tobacco products by young persons.
Description
The Regulations apply to vaping products that are intended to be sold at retail or otherwise furnished at a point of sale that is a retail establishment where vaping products are ordinarily sold in Canada, as well as to their packaging.
The Regulations establish a maximum nicotine concentration of 20 mg/mL for vaping products. This measure applies to manufacturers, including importers, of vaping products. Any manufacturer that manufactures or sells a vaping product that does not conform with this limit will be in contravention of section 7.2 of the TVPA. This will not affect the supply of nicotine to manufacturers of vaping products and the export of vaping products. Vaping products authorized under the FDA are not subject to the Regulations.
The Regulations prescribe the laboratory method entitled “ISO 20714 E-liquid — Determination of nicotine, propylene glycol and glycerol in liquids used in electronic nicotine delivery devices — Gas chromatographic method” for determining the nicotine concentration of a vaping substance.footnote 19Health Canada will use this method to determine compliance with the maximum nicotine concentration. This method could be used by manufacturers and importers who wish to assess if their products meet the prescribed nicotine standard. Since the ISO method provides results expressed in mg/g, the Regulations describe how to convert them into mg/mL.
The Regulations also prohibit the packaging and sale of vaping products where the package displays, in the nicotine concentration statement required by the VPLPR, a value that exceeds 20 mg/mL. This measure applies to any person who packages or sells vaping products in Canada.
Pursuant to the VPLPR, the manufacture, importation, advertisement or sale of vaping products containing 66 mg/mL or more of nicotine is prohibited. The Regulations amend the VPLPR to ensure alignment between both sets of regulations for vaping products manufactured or imported for sale in Canada. The amendment maintains the maximum nicotine concentration of 66 mg/mL or more for products intended for export.
The Regulations come into force on the 15th day after the day on which they are published in the Canada Gazette, Part II. Retailers have a transition period of 15 days, following the coming-into-force date, during which they will be allowed to continue to sell a vaping product that is packaged in a manner that is contrary to these Regulations.
Regulatory development
Consultation
Reducing Youth Access and Appeal of Vaping Products: Consultation on Potential Regulatory Measures
The consultation document entitled Reducing Youth Access and Appeal of Vaping Products: Consultation on Potential Regulatory Measures (PDF) was published on the Government of Canada website on April 11, 2019, providing a 45-day comment period that closed May 25, 2019. The consultation asked Canadians for input on various measures to reduce youth access to vaping products and to reduce the appeal of vaping products to youth. One of the measures proposed was to reduce the nicotine content in vaping products.
Health Canada received nearly 23 000 postcards from people who vape who were opposed to additional measures and who reported either quitting smoking with vaping or attempting to quit smoking with vaping. Most of these respondents also provided information regarding the nicotine concentrations they used. In total, 87% reported using a concentration of 20 mg/mL or less, with the most common concentrations reported being 3 mg/mL (32% of respondents) and 6 mg/mL (30% of respondents). Also, 2% reported using nicotine-free products and 3% reported using vaping products that contained more than 50 mg/mL of nicotine.
Excluding postcard respondents, Health Canada received 288 comments from stakeholders in response to the consultation. Approximately 50% of respondents expressed support for further restrictions on nicotine concentration, 13% were opposed, and 37% were unclear or provided no opinion.
Respondents who supported lowering the maximum nicotine concentration in vaping products included parents, educators, the general public, non-governmental organizations (NGOs), health authorities, provinces and territories, and a small number of specialty vape shop owners and operators. Some of the suggestions and comments received from these stakeholders included the following:
- Restrict the manufacture and sale of vaping products to nicotine concentrations of
- 20 mg/mL and lower, in line with the European Union,
- 24 mg/mL and lower (reported by an association of specialty vape shop owners as the highest concentration they sell), or
- 36 mg/mL and lower (reported by some stakeholders as highest level sold in Canada prior to 2018);
- Prohibit the manufacture and sale of vaping products with nicotine in salt form or restrict available concentrations of nicotine in salt form in vaping products;
- Restrict the manufacture and sale of flavoured vaping products to nicotine concentrations of 20 mg/mL and lower. Some respondents suggested only allowing higher concentrations of nicotine in vaping products with tobacco flavours;
- Restrict the sale of high-nicotine-concentration products to specialty vape shops and other age-controlled locations; and
- Restrict the sale of all nicotine-based vaping products to specialty vape shops and other age-controlled locations.
These stakeholders also suggested an illegal market could result from lowering the maximum allowable concentration of nicotine, but asserted that the benefits of more regulation would outweigh this potential risk.
Many specialty vape shop owners and operators and their associations mentioned that lowering the nicotine concentration may result in fewer adults who smoke switching to vaping. However, a few specialty vape shop respondents suggested there may be a level above which additional nicotine is not necessary, and that there are reasonable rationale and justification to restrict certain extreme concentrations. It was suggested that placing a restriction on the concentration of nicotine could potentially minimize the risks of nicotine dependence, nicotine toxicity or unintentional overconsumption among youth and non-smoking adults and help mitigate a transition from vaping to smoking. Although some of these stakeholders were supportive of the regulatory proposal to lower the concentration of nicotine in vaping products, many also suggested the current TVPA restrictions, such as those prohibiting the promotion of certain vaping product flavours and the promotion and sale of vaping products that are appealing to young people, be more properly enforced.
Gas and convenience (G&C) store owners and operators and their associations, and some specialty vape shop owners, were not supportive of any further restrictions to nicotine concentration in vaping products. These stakeholders mentioned that current TVPA restrictions are sufficient and that the TVPA provides adequate protection against overtly youth-oriented products. They also emphasized their role as responsible and experienced retailers with their own codes of conduct for keeping restricted products out of the hands of minors. Most of these industry respondents voiced concerns about possible unintended consequences of overly restrictive regulations that will decrease the ability of vaping products to compete with cigarettes, decreasing the likelihood of adults who smoke successfully switching to vaping products. Moreover, these respondents felt strongly that if enacted, any further restrictions should be applied consistently across all retail channels to create a level playing field and should not provide specialty vape shops with an unfair competitive advantage.
Health Canada's response to key stakeholder concerns
Location of sale of vaping products
A few specialty vape shop owners and operators suggested the sale of high-nicotine-concentration vaping products or all vaping products containing nicotine be restricted to adult-only specialty stores. Moreover, some of them suggested people who smoke may need a higher nicotine concentration to help them transition to vaping products. G&C store owners and operators and their associations suggested that restrictions be applied consistently across all retail channels.
Response: Setting a maximum nicotine concentration for all vaping products sold on the Canadian market restricts the possibility of high-nicotine-concentration products getting into the hands of young persons. Vaping products with nicotine levels similar to those available to adults who smoke prior to the rapid increase in youth vaping will remain on the market. Restrictions on where products can be sold generally fall under provincial jurisdiction. The Regulations do not preclude provinces limiting where vaping products can be sold.
Restricting nicotine concentration in flavoured vaping products
Stakeholders supportive of further measures to restrict nicotine in vaping products, including health authorities, provinces and territories and the vast majority of NGOs, suggested Health Canada prohibit or restrict the manufacture and sale of vaping products with flavours above a certain threshold of nicotine concentration (20 mg/mL was commonly identified), or that the manufacture and sale of higher nicotine concentration vaping products be restricted to only tobacco flavours.
Response: High concentrations of nicotine in vaping products are appealing to youth. Leaving high-nicotine-concentration products on the market, even only in certain flavours, is expected to continue to act as an inducement for youth to use these products. Potential regulatory options to restrict the manufacture and sale of vaping products with certain flavours are under consideration by Health Canada.
Nicotine salts
Stakeholders supportive of further measures to restrict nicotine in vaping products suggested prohibiting the manufacture and sale of nicotine salts or restricting their sale to lower concentrations of nicotine.
Response: The Regulations will limit the concentration of nicotine regardless of its forms (i.e. salt and non-salt forms). Only prohibiting the use of nicotine salts would not prevent high-nicotine-concentration products from remaining on the market, although they would be in a form that is more aversive to nicotine-naive users. With respect to monitoring compliance with a ban on the use of nicotine salts, Health Canada is not aware of reliable laboratory methods to directly identify the form of nicotine used in a given vaping substance. Health Canada will continue to monitor the research and development in this area (see the “Instrument choice” section below).
Nicotine concentration limit for vaping products
Stakeholders supportive of further measures to restrict nicotine (parents, educators, the public, public health NGOs, health authorities, provinces and territories, some specialty vape shop retailers) expressed concerns with the high-nicotine-concentration vaping products currently on the market. Their suggestions varied from establishing a maximum nicotine concentration limit of 36 mg/mL to eliminating nicotine in vaping products.
Response: The measures introducing a nicotine concentration limit of 20 mg/mL align with the limits in force in several other jurisdictions and with the views expressed by a majority of stakeholders. This limit is also consistent with the maximum levels of nicotine found in the majority of vaping products sold on the Canadian market prior to the observed rapid increase in vaping product use by youth.
Prepublication in the Canada Gazette, Part I
The proposed Regulations were prepublished on December 19, 2020, in the Canada Gazette, Part I, for a 75-day consultation period that ended on March 4, 2021.
A total of 3 874 responses were received during the consultation period, including 3 569 from five distinct letter-writing campaigns and 305 individual responses. The individual responses were comprised of 171 submissions from the general public, 48 from the vaping industry (including 15 manufacturers, 28 specialty vape shops, 3 vaping industry associations), 1 from a retailer association, 1 from a gas and convenience store, 22 from health care providers, 16 from NGOs, 15 from public health authorities, 9 from harm reduction advocates, 8 from business associations, 5 from academics, 4 from associations of education professionals, 4 from associations of health professionals and 2 from provincial and territorial governments and 1 from a municipal government. The letter-writing campaigns sent in 2 036 submissions opposed to the proposal and 1 533 submissions in support of the proposal.
Those who opposed the proposal were from the vaping industry (specialty vape shops, vaping industry associations), retailer associations, business associations, harm reduction advocacy, academia and the general public, including many who identified themselves as vapers. Their key concerns are summarized below with Health Canada's responses. Those who supported the proposal were from NGOs, health care providers, associations of health professionals, associations of education professionals, public health advocacy and the general public. They supported the objective of the Regulations to protect youth from the harms of vaping, including risk of dependence that could result from using nicotine containing vaping products. All comments were given due consideration.
Several submissions included comments which were outside the scope of the regulatory proposal, such as those relating to flavour restrictions in vaping products and measures to further limit youth access to vaping products. Health Canada will consider them in the development of future proposals.
Health Canada's response to key stakeholder concerns
Youth vaping and tobacco use
Vapers, specialty vape shops, harm reduction advocates and academics challenged the assertion that youth vaping could lead to an increased risk of tobacco use, often referred as the “gateway effect” by many respondents.
Response: The NASEM report published in 2018 concluded that there is “substantial evidence that e-cigarette use increases the risk of ever using combustible tobacco cigarettes among youth and young adults.”footnote 7 Several longitudinal studies have reported a positive association between adolescent vaping and subsequent initiation of cigarette smoking. This positive association is also present among “low-risk” adolescents (i.e. adolescents who are not at high risk for cigarette smoking based on established risk factors).footnote 20 Studies have shown that vaping product use by youth increases the frequency and intensity of subsequent combustible tobacco cigarette smoking.footnote 21 Symptoms of dependence among young vaping product users have been observed, including frequency of use, experiencing frequent strong urges to vape, feelings of addiction and unsuccessful quit attempts.footnote 22 These findings support public health concerns that vaping may contribute to the development of a new generation of people who vape and smoke cigarettes. The Regulations, along with other measures, are expected to reduce inducements to youth that promote experimentation with vaping products.
Benefits analysis
The vaping industry, harm reduction advocates and academics did not agree with the methodology used by Health Canada to evaluate the benefits of the Regulations, which associated a decrease in youth inducements to use vaping products with a decrease in the risk of smoking initiation. They also disagreed with the estimates used to qualify the harm of vaping products compared to tobacco products, which was estimated at 20% of the relative harm of tobacco.
Response: Based on available research, youth who use vaping products are more at risk of initiating cigarette smoking compared to those who do not vape. There is currently no data available on the long-term health consequences of vaping due to the relatively recent use of these products. Tobacco-related morbidity and mortality typically occur decades after smoking initiation due to the repeated and long-term exposure to the harmful chemicals in tobacco smoke. Until decades of epidemiological data related to vaping product use is available, accurate estimates of their harm cannot be made. This also applies to comparisons of long-term health effects between vaping products and tobacco products.
The benefits analysis addresses these limitations by using a break-even analysis that specifies the reduction in the vaping initiation rate over the next 30 years that would be necessary to produce public health benefits commensurate with the costs of the Regulations. The benefits were calculated against the costs associated with lifelong tobacco use that could result from youth vaping and an addiction to nicotine. Economic costs associated with tobacco-related morbidity, including health care costs and short-term disability, are available in the literature due to the known long-term health consequences of smoking and were used for the analysis.
Recognizing that the Regulations may adversely affect the rate at which adults who smoke switch completely to vaping products, a sensitivity analysis was used to examine how this might influence the break-even analysis. Due to the absence of data on the long-term health consequences of vaping, the analysis used expert opinion to set the harms of vaping at a 20% fraction of the health consequences of cigarette use. Health Canada believes that the current benefits analysis approach, which estimates the public health consequences of changes in Canadians' use of tobacco products and vaping products, is appropriate in this context.
Vapers will be exposed to more harmful chemicals
Vapers, harm reduction advocates and academics were concerned that the Regulations would result in some vaping product users inhaling more harmful substances to compensate for lower levels of nicotine available in vaping products, as a result of the Regulations.
Response: Short-term studies have observed that vapers who switch from high-nicotine-concentration vaping products to lower nicotine concentrations compensate for the change in nicotine level by modifying their puff frequency, duration or volume of inhaled vapour to obtain the desired nicotine level. This change in vaping behaviour could expose them to more harmful chemicals; the difference in exposure cannot be quantified. It is unclear if new vaping product users compensate in the same manner in response to the available nicotine concentration or whether those who switch from higher nicotine concentrations maintain their modified patterns of use over longer periods of time. The amount of chemicals and contaminants in vapour is normally at much lower levels than in cigarette smoke. Vaping products that contain less than 20 mg/mL of nicotine would remain a less harmful alternative for people who smoke who are unable or unwilling to quit nicotine if they switch completely to vaping.
Although we are learning more about how vaping affects health, the long-term health impacts are still unknown. However, there is enough evidence to justify efforts to prevent the use of vaping products by young persons and non-users of tobacco products.
Lower nicotine concentrations will limit the ability of adults who smoke to switch completely to vaping products
Vaping industry associations, specialty vape shops, harm reduction advocates, academics and vapers commented that removing high-nicotine-concentration vaping products would make it more difficult for people who smoke who wish to transition away from smoking. They also mentioned that some former smokers who currently vape high-nicotine-concentration products would relapse to smoking.
Response: Health Canada previously identified that the Regulations may cause some vapers to return to smoking if they are unable to transition to lower nicotine levels at or below the 20 mg/mL. However, vaping products that contain 20 mg/mL nicotine or below could still assist people who smoke to transition to vaping. This is consistent with results from clinical trials, conducted in the United Kingdom, that suggest vaping products with less than 20 mg/mL of nicotine increase quitting success compared to smoking cessation aids containing nicotine when both products were accompanied by behavioural support.footnote 23 Other alternatives also exist to help people who smoke to quit tobacco. Health Canada has authorized a number of smoking cessation aids with nicotine, including nicotine gum, nicotine patches, nicotine inhalers, and nicotine lozenges. Authorization under the FDA was delivered after a review of their efficacy, safety and quality. Vaping product manufacturers can seek market authorization under the FDA for vaping products that contain nicotine above 20 mg/mL; to obtain such authorization, they would have to make a therapeutic claim and provide substantive scientific evidence of efficacy, safety and quality.
Setting a maximum nicotine concentration at 20 mg/mL will lead to illicit market demand for these products
Vaping industry associations, specialty vape shops and vapers who oppose the 20 mg/mL nicotine concentration limit suggested that people may seek out high-nicotine-concentration products from foreign online suppliers or the illicit market.
Response: As a result of the amendment to the VPLPR, vaping products that are ordered online from other countries or otherwise imported into Canada will be subject to the lower limit of 20 mg/mL, but not if they are imported for export. Imported vaping products could be subject to an inspection by the Canada Border Services Agency and non-compliant products could, among other things, be seized or returned to the sender. Health Canada inspectors will also be monitoring the market place; non-compliant (illicit market) products will be subject to appropriate enforcement action.
Implementation period of 15 days following publication of the Regulations in the Canada Gazette, Part II
A number of specialty vape shops, manufacturers of vaping products, vaping industry associations and business associations commented that the proposed 15-day implementation period, after publication of the Regulations in the Canada Gazette, Part II, was too short. Some of these responses did not take a position on the nicotine limit proposed and were only concerned about the short implementation period. Vaping product retailers commented that they would need more time to dispose of the non-compliant products. Vaping product importers and industry associations requested that the coming-into-force period be extended to a period of 180 days to give them more time to eliminate their existing stock of vaping products that are above 20 mg/mL nicotine. Businesses mentioned that financial impacts of the COVID-19 pandemic measures should also be taken into consideration. Stakeholders commented that the 15-day implementation period does not meet Canada's international obligations with regards to trade and that it sets a bad precedent for the regulation of consumer products. Stakeholders in favour of the proposal, which included NGOs, public health advocates and the general public, supported a short implementation period and wanted the Regulations to be implemented as soon as possible (“on an urgent basis”) to protect Canadian youth from vaping product inducements.
Response: The 15-day implementation period after publication in the Canada Gazette, Part II, has been maintained for manufacturers and importers, with the addition of a transitional period for retailers. Foreign and Canadian manufacturers already supply vaping products at or below 20 mg/mL of nicotine; therefore, they do not need additional time to be in compliance with the Regulations. The short implementation period was deemed necessary to help address youth vaping, which Health Canada considers to be an urgent health problem.
In consideration of the concerns expressed by specialty vape shops, G&C stores and retailer associations that the 15-day implementation period did not provide enough time for retailers to dispose of non-compliant products, Health Canada has added a transitional period of 15 days for retailers only. This provides retailers with a total of 30 days after the day the Regulations are published in the Canada Gazette, Part II, to comply with the prohibition on packaging and selling vaping products that display a statement showing a nicotine concentration above 20 mg/mL.
An implementation period beyond 30 days was considered to undermine the objectives of these Regulations.
Modern treaty obligations and Indigenous engagement and consultation
The Regulations are not expected to impact modern treaties with the Indigenous peoples of Canada. Analysis regarding possible differential impacts on Indigenous peoples is set out in the “Gender-based analysis plus” section below.
Instrument choice
Option 1: Baseline scenario (no further restriction on the nicotine concentration)
This option would maintain the existing legislative regime with respect to regulating the concentration of nicotine. Section 49 of the VPLPR sets out that a vaping product must not contain nicotine in a concentration of 66 mg/mL or more. This concentration limit for nicotine is based on a peer-reviewed toxicity evaluation of the ingestion of pure nicotine. The limit is consistent with how a very toxic consumer chemical is differentiated in the Consumer Chemicals and Containers Regulations, 2001.
This option would leave vaping products with nicotine concentrations that are appealing to young persons, thus maintaining a risk of further vaping uptake by nicotine-naive users, particularly youth.
Therefore, the status quo is not considered to be an appropriate option.
Option 2: Standardize the permitted form of nicotine in vaping products
This option would prohibit the manufacture and sale of vaping products that contain nicotine salts.
This option would potentially reduce the appeal of vaping products, as the harshness of nicotine (i.e. not in its salt form) may render vaping products more aversive to nicotine-naive users, particularly young persons experimenting with vaping products. This option would still potentially leave high-nicotine-concentration vaping products on the market, although not in salt form. These products are expected to still be appealing to young persons seeking the “head-rush” or “buzz” described by some novice users.
Regarding its ability to monitor compliance with such a ban, Health Canada is not aware of reliable laboratory methods to directly measure the form of nicotine used in a given vaping substance.
Option 3 (recommended): Setting a maximum nicotine concentration in vaping products
This option sets a maximum nicotine concentration for vaping products. The limit of 20 mg/mL is expected to reduce the appeal of vaping products to young persons and potentially reduce the likelihood that young persons will experiment with or continue to use these products, which may also lead them to tobacco use. It would apply to all vaping products regardless of the type of nicotine, including those in salt form. The availability of a validated international test method (ISO 20714) would provide clarity to regulated parties to help them comply with this option.
The maximum nicotine concentration selected will still allow adults who smoke access to vaping products as a less harmful alternative to cigarettes if they switch completely to vaping. The majority of older adults use vaping products below 20 mg/mL nicotine and this was the case even prior to the observed increase in youth vaping, as vaping products were mostly available in those concentrations prior to 2018.
The maximum nicotine concentration aligns with the 20 mg/mL limit in place in the European Union's 27 Member States, the United Kingdom, Iceland, Israel, Moldova and Saudi Arabia. This option also aligns with the maximum nicotine concentration set for vaping products sold at retail stores in the provinces of British Columbia and Nova Scotia.
This option is recommended because it supports the objective of protecting young persons from inducements to use vaping products. Health Canada recognizes that young persons could still be attracted to vaping products below 20 mg/mL nicotine and that other measures may be needed to further address the appeal of vaping products.
Regulatory analysis
Benefits and costs
Summary of cost-benefit analysis
The Regulations are expected to contribute to reducing the appeal of vaping products to young persons to protect them from inducements to use vaping products.
The Regulations will result in total incremental costs for the vaping industry that are estimated at $452.0 million, expressed as present value (PV), over 30 years (or about $36.4 million in annualized value). The monetized costs to the vaping industry are associated with the disposal of stocks of vaping products above 20 mg/mL nicotine, which would no longer be sold or distributed, and potential industry profit losses.
Implementation of the Regulations will require a minimal investment of public sector resources. There will be a small one-time cost of $18,682 PV over 30 years (or about $1,506 in annualized value) to Health Canada relating to the publication of compliance promotion material, including a notice in trade magazines and the addition of information on the new Regulations to the Government of Canada website. Aside from this cost, there will be no incremental costs for Health Canada in performing compliance and enforcement activities. Monitoring compliance with the Regulations will involve activities such as sampling of vaping products, their testing for nicotine concentration and review of information on their labels; these activities are already in place at Health Canada to monitor compliance with the VPLPR.
The Regulations will support the CTS, which aims to reduce the burden of disease and death caused by tobacco use and its consequential impact on the public health care system and society. The Regulations are expected to primarily benefit youth by contributing to the reduction in the number of those who experiment with vaping products, which can lead to exposure to and dependence on nicotine and transition into tobacco use. Long-term economic benefits would be realized in terms of avoided tobacco-related mortality and morbidity, including exposure to second-hand smoke. The break-even analysis indicates that a decrease in the rates of vaping initiation in the range of 2.58% to 4.11% relative to the baseline initiation rate would be sufficient to produce public health benefits equivalent to or greater than the estimated monetized costs.
Analytical approach
The Cabinet Directive on Regulation requires departments to analyze the costs and benefits of federal regulations. To measure these impacts, the benefits and costs are estimated by comparing the incremental change from the current regulatory framework (i.e. the “baseline scenario”) to what is anticipated to occur under the new regulatory approach (i.e. the “regulatory scenario”). The Regulations will come into effect in 2021. This cost-benefit analysis (CBA) covers the 30-year period from 2021 to 2050. A 7% discount rate is used to estimate the present value of the incremental costs and incremental benefits. All values reported for the 30-year period are expressed in 2019 constant dollars.
The regulatory impacts in this analysis have been estimated using three approaches: quantitative analysis where possible, qualitative analysis, and a break-even analysis. The costs analysis incorporates information gathered from representatives of the vaping industry. A summary of the CBA is provided herein. A copy of the CBA report is available upon request from hc.pregs.sc@canada.ca.
Overview of the vaping industry in Canada
The overall vaping product market in Canada was estimated at $1.36 billion in 2019. There are approximately 200 vaping liquid manufacturers in Canada and 15 to 20 large distributors. Canadian importers of vaping liquids and devices obtain their supplies (devices and raw materials / ingredients, including nicotine and flavours) mostly from the United States and China. Between 85% and 95% of the total volume of vaping liquid sold in Canada is manufactured in Canada. The 50 largest manufacturers account for about 80% of this share. Vaping liquid sold in bottles is almost exclusively manufactured in Canada, while vaping liquid sold in pre-filled pods is almost exclusively imported into Canada. Bottled liquid outsold pod liquid by a factor of at least 7 to 1 in terms of volume in 2019. Contract manufacturing of vaping substances (i.e. vape shops using the services of a laboratory to manufacture their vaping liquids) is common in Canada.footnote 24,footnote 25,footnote 26
Vaping products are sold in three main categories of stores: vape shops, G&C stores and online retailers. The market breakdown by channel based on value is as follows: 49% in vape stores, 30% in G&C stores, 21% online. There are 1 400 vape stores, 25% of which are chained retailers, as well as 27 240 G&C stores, 37% of which are chained retailers, and about 1 500 websites, most of which are the online retail component of brick-and-mortar stores. The majority of these businesses, including manufacturers, are considered to be small under the Treasury Board of Canada Secretariat definition.footnote 27,footnote 25,footnote 32,
Overview of vaping product users in Canada
Data from the 2020 CTNS show the prevalence of past-30-day vaping was 13% among young adults aged 20 to 24, and 3% among adults aged 25 years and older. Furthermore, never smokers made up the majority of past-30-day vape users within youth aged 15 to 19 (74%). This contrasts with young adults aged 20 to 24 and adults 25 years and older where the majority of past-30-day vapers were either current or former smokers, at 46% and 94%, respectively.footnote 33,
Assessment of costs and benefits
It is anticipated that the Regulations will impact youth, people who smoke or vape and the vaping industry in all provinces and territories, except for British Columbia and Nova Scotia. These two provinces have regulations in place that set a maximum nicotine concentration at 20 mg/mL in vaping products. In Ontario, only specialty vape stores will be impacted by the Regulations since provincial rules restrict the sale of vaping products with nicotine concentrations greater than 20 mg/mL to these stores. The Regulations will also impact the Government of Canada.
Costs of the Regulations
Quantitative costs
1. Costs to vaping industry associated with the disposal of stocks of vaping products with a nicotine concentration above 20 mg/mL
The Regulations set a maximum nicotine concentration of 20 mg/mL for vaping products intended to be sold at retail or otherwise furnished at a point of sale that is a retail establishment where vaping products are ordinarily sold in Canada. These measures only apply to manufacturers, including importers, of vaping products. They do not affect the supply of nicotine to manufacturers of vaping products and the export of vaping products.
Manufacturers and importers of vaping products across Canada, excluding British Columbia and Nova Scotia, that sell vaping products above 20 mg/mL nicotine will be impacted by the Regulations because they will be unable to distribute or sell their remaining stock in the 15-day period after publication of the Regulations in the Canada Gazette, Part II. It is assumed that all these businesses will dispose of all of their remaining stock of vaping products above 20 mg/mL nicotine. A one-time incremental cost would be carried by these manufacturers and importers. This cost is calculated based on the market value of the remaining stocks. As a result, it is acknowledged that the disposal cost may be an overestimate because this cost includes manufacturer profits, which cannot be materialized, as the non-compliant products will be disposed of.
The one-time incremental cost associated with the disposal of the remaining stocks of vaping products above 20 mg/mL nicotine is estimated at $58,254,497 PV over 30 years (or about $4,694,520 in annualized value). This cost is assumed to occur in 2021.
2. Costs to vaping industry in terms of profit loss
It is anticipated that the vaping industry will experience a loss of sales to adult customers who choose to discontinue using vaping products rather than transition to vaping products that contain 20 mg/mL nicotine or below.
After implementation of the Regulations, manufacturers and importers who are currently manufacturing, distributing and selling vaping products above 20 mg/mL nicotine will potentially incur incremental costs in terms of profit loss resulting from the loss of market sales. It is anticipated that retailers will also incur profit loss as they will no longer be permitted to sell these products to consumers once the Regulations come into force.
It is also anticipated that fewer youth would experiment with vaping products as a result of their reduced appeal, and thus fewer youth would transition to adult vapers. Most young persons obtain vaping products illegally or from social sources, i.e. from adults or other youth.
In the baseline, it is projected that the compound annual growth rate of sales of vaping products is 15% from 2021 to 2024 based on historical data.footnote 28 It is further assumed that the overall growth rate of sales of vaping products is 0% during the 2025–2050 period (the analytical horizon). The 0% growth rate assumptions from 2025 to 2050 is based on recent sales projections of the vaping market in Canada. This takes into account federal and provincial/territorial governments advancing a number of regulatory interventions to address youth vaping as well as investing in marketing campaigns to warn Canadians, especially youth, about the harms of vaping. These interventions are expected to make vaping products less appealing to youth and thus limit sales growth. Furthermore, the prevalence of past-30-day use of vaping products among adults (aged 25 and older) has been stable since 2015 at around 2% (Canadian Tobacco, Alcohol and Drugs Survey [CTADS] 2015, 2017 and CTNS 2019).footnote 29
For the purpose of this analysis, it is assumed that the number of vapers is proportional to market size (in value). Furthermore, it is assumed that vapers who transition from vaping products above 20 mg/mL to 20 mg/mL nicotine or below will consume, on average, the same amount of vaping product as an average person vaping with products at 20 mg/mL nicotine or below. It is also assumed that approximately 75% of current users of vaping products above 20 mg/mL nicotine will switch to vaping products at 20 mg/mL nicotine or below after implementation of the Regulations.footnote 30 It is also assumed that those who switch to vaping products at 20 mg/mL nicotine or below after the Regulations come into force, would maintain their consumption level, so that 75% of the market sales (in dollar value) of vaping products above 20 mg/mL nicotine in the baseline will be transferred to the market sales (in dollar value) of vaping products below 20 mg/mL. It is further assumed that those persons would remain in the market of vaping products at 20 mg/mL nicotine or below over the analytical period.
It is estimated that the profit margin ratio of vaping products above 20 mg/mL nicotine is about 42.27% and that of vaping products of 20 mg/mL nicotine or below is about 51.43%.footnote 31Profit loss would be mitigated by the proportion of adult consumers who use vaping products above 20 mg/mL nicotine who transition to vaping products at 20 mg/mL nicotine or below. It is expected that the vaping industry profit losses would be offset as a result of this. Therefore, it is anticipated that the total profit loss to the vaping industry would be equal to the profit loss due to the loss in sales of vaping products above 20 mg/mL nicotine, minus the profit gain as a result of vapers transitioning to vaping products at 20 mg/mL nicotine and below after the coming into force of the Regulations.
The estimated potential profit loss is $338,776,267 PV over 30 years (or about $27,300,761 in annualized value) for manufacturers and importers, and $54,961,710 PV over 30 years (or about $4,429,167 in annualized value) for retailers. In total, the incremental costs in terms of potential profit loss are estimated at $393,737,977 PV (or about $31,729,927 in annualized value) for all businesses in the vaping industry. The incremental costs on companies are the profit margin after taxes, which is equal to the revenue of the company minus the costs of goods sold. The costs of operation and debts are also included in the estimation. There could be some minor tax revenue loss to governments as a result of the Regulations given that vaping products above 20 mg/mL nicotine will be removed from the market.
The cost impact analysis for the Regulations was calculated using market data obtained prior to the changes implemented in Ontario that limited the sale of vaping products with over 20 mg/mL of nicotine to specialty vape stores. For the purpose of the cost calculations, it is assumed that the market value related to the sales of vaping products with over 20 mg/mL of nicotine that was previously permitted in other locations, including G&C stores, would have shifted to specialty vape stores by the time the Regulations come into force. If the market value of those sales did not completely shift to specialty vape stores (i.e. if consumers shifted to lower concentration vaping products in Ontario), then the CBA would have overestimated the costs by up to 10%, which would have resulted in a lower break-even requirement.
Sensitivity analysis
A sensitivity analysis examined the uncertainty of variables and how they may affect cost results. Two qualified variables were selected for the sensitivity analysis, namely the profit margin ratio and the estimated percentage of adult vapers consuming vaping products above 20 mg/mL nicotine in the baseline who would switch to vaping products with 20 mg/mL nicotine or below after the Regulations come into force (switch rate). After an initial screening of the two variables, the switch rate had the most impact on the costs results and was therefore selected as a variable for the sensitivity analysis.
There is uncertainty on the estimated percentage of adult vapers using vaping products above 20 mg/mL nicotine in the baseline who would switch to vaping products with 20 mg/mL nicotine or below after the Regulations come into force. Data suggests the majority of adult vapers are already using vaping products with a nicotine concentration below 20 mg/mL in the baseline (see “Background” section). It is assumed that 100% of adult vapers consuming vaping products above 20 mg/mL nicotine in the baseline would switch to vaping products with 20 mg/mL nicotine or below after the Regulations come into force. A switch rate of 50% was assumed in order to address the uncertainty of this variable. The switch rates of 75%, which is the mean value of two scenarios (100% and 50%), and 50% were chosen for the sensitivity analysis.
At a switch rate of 100%, there would be no incremental cost impact on the vaping industry. Since it is more profitable for the vaping industry to manufacture vaping products with 20 mg/mL nicotine or below, it is anticipated that the vaping industry could in fact benefit from the Regulations assuming a 100% switch rate. At a switch rate of 50% (high-cost scenario), the cost impact would be $1,136,061,694 PV over 30 years (or about $91,551,126 in annualized value). The switch rate of 75% (low-cost scenario) was used in the analysis. As presented in the cost-benefit statement, the 75% switch rate would result in an incremental cost of $451,992,474 PV over 30 years (or about $36,424,448 in annualized value).
Total incremental costs (shown as a range) to the vaping industry were estimated over 30 years as $451,992,474 to $1,136,061,694 (PV) [or about $36,424,448 to $91,551,126 in annualized values].
3. Costs to Government (Health Canada) associated with implementation, enforcement and compliance activities
Implementation of the Regulations will require a minimal investment of public sector resources.
Implementation activities would include making minor revisions to current laboratory procedures to include the testing of samples of vaping products to determine whether the nicotine concentration exceeds 20 mg/mL. Health Canada already has testing procedures and laboratory facilities to support VPLPR-related compliance and enforcement activities. No additional investment in laboratory testing equipment would be needed.
It is expected that Health Canada will incur a one-time implementation cost to produce compliance promotion material, including a notice in trade magazines and the addition of information on the new regulations to the Government of Canada website. The total government cost is estimated at $18,682 PV over 30 years (or about $1,506 in annualized value).
Aside from the cost to produce the compliance promotion material, there will be no incremental costs for Health Canada to conduct compliance and enforcement activities associated with the Regulations. To monitor compliance with the maximum nicotine concentration limit of 20 mg/mL, Health Canada inspectors will collect samples at manufacturers' and importers' facilities for testing at Health Canada laboratories. Given that such sampling and testing are already in place to monitor compliance with the VPLPR, there will be no additional costs to Health Canada. Travel costs relating to the collection of samples of vaping products are not expected to increase as Health Canada inspectors are already involved in this type of travel as part of VPLPR-related activities. Inspectors' review of labels to determine if the stated nicotine concentration exceeds 20 mg/mL will also become part of existing VPLPR-related activities.
Qualitative costs
4. Costs to vaping industry as a result of dual users modifying their smoking behaviour
About 46% of current vapers (past-30-day use) aged 20 years and older are dual users (i.e. individuals who vape and smoke cigarettes).footnote 34 Total profit loss to vaping industry members who are also manufacturers of tobacco products may be mitigated by substitution of tobacco purchases from dual users who would go back to smoking and adults who smoke who would continue to smoke instead of switching to vaping products at 20 mg/mL nicotine or below.
After the Regulations come into force, it is anticipated that some dual users who currently use vaping products above 20 mg/mL nicotine would not substitute their vaping product purchases with lower concentrations of nicotine. They would choose to purchase more cigarettes, hence offsetting the loss of sales of vaping products above 20 mg/mL nicotine. However, Health Canada does not have the data necessary to quantify these costs.
5. Costs to retailers as a result of the packaging and sales prohibition
The Regulations also prohibit the packaging of vaping products in a package that displays a statement indicating a nicotine concentration above 20 mg/mL, as well as the sale of vaping products packaged this way. These measures apply to any person, including manufacturers, importers, distributors and retailers of vaping products, that packages or sells vaping products in Canada.
The cost impact of this provision for manufacturers and importers has already been captured in the section “Costs to vaping industry associated with the disposal of stocks of vaping products with a nicotine concentration above 20 mg/mL.” With regard to retailers, it is assumed that any remaining stock of vaping products above 20 mg/mL nicotine will be returned to suppliers once the Regulations come into force.
6. Costs to adults who smoke and dual users
There may be some incremental cost impacts on adults who smoke and adult dual users who use vaping products above 20 mg/mL nicotine. Some current smokers who would try vaping products may find that vaping products at 20 mg/mL nicotine or below are not satisfying to them and could therefore end up being dual users or remain smokers. These persons would continue to be exposed to harmful chemicals from the long-term use of tobacco products.
It is also anticipated that certain dual users could relapse to smoking only as a result of the Regulations. However, benefits of vaping by people who smoke are only accrued if they completely switch to vaping.
Overall, if people who smoke do not completely switch to vaping, long-term benefits would not be realized in terms of avoided tobacco-related mortality and morbidity, and exposure to second-hand smoke. These costs were considered when performing the sensitivity analysis that examined the break-even points where the reduction in vaping initiation rate provides benefits that equal the costs of the Regulations.
Benefits of the Regulations
The Regulations support the CTS, which aims to reduce the burden of disease and death from tobacco use and its consequential impact on the public health care system and society. The success of the CTS, a federal initiative, will be a result of a multifaceted and coordinated approach and the tobacco control efforts of many partners, such as provinces and territories, municipalities, non-governmental organizations, community agencies and the private sector. Given the variety and number of tobacco control interventions at play, quantifying the benefits of an individual tobacco control measure is very challenging.
The Regulations will primarily benefit youth by contributing to the reduction in the number of young persons who experiment with vaping products, which can lead to exposure to and dependence on nicotine and transition into tobacco use. Long-term benefits would be realized in terms of avoided tobacco- and vaping-related mortality and morbidity, including from exposure to second-hand smoke. Given the significant uncertainties associated with the expected impact of the Regulations on vaping prevalence, the direct public health benefits attributable to the Regulations were not monetized. Instead, a model was developed to examine the implications of changes in vaping initiation rates on fatal and non-fatal health effects of tobacco and vaping product use. Three benefits resulting from changes in the initiation rates were considered, namely
- (1) reduced tobacco- and vaping-related mortality;
- (2) reduced tobacco- and vaping-related morbidity; and
- (3) reduced exposure to second-hand smoke.
There is currently no data on the long-term health consequences of vaping due to the relatively recent use of these products in Canada. For example, tobacco-related morbidity and mortality typically occur decades after smoking initiation due to the repeated and long-term exposure to the harmful chemicals in tobacco smoke. The model was therefore designed to express the health consequences of long-term vaping product use as a fraction of the health consequences of conventional cigarette use based on the opinions of health professionals.
Model description
The model was used to conduct a break-even analysis to determine the percentage reduction of the initiation rate of vaping products over the next 30 years that would need to occur in order to provide public health benefits that are equal to or exceed the estimated costs. Furthermore, recognizing that the Regulations may adversely affect the rate at which adults who smoke switch to vaping, a sensitivity analysis was conducted on the benefit analysis to examine how switch rates might influence the break-even point.
Tobacco-related mortality
To estimate the mortality risk of current and former smokers, data from Taylor et al.footnote 35 on mortality risks as a function of sex, age, and time since quitting was relied upon. The estimates were adjusted so that the model's aggregate age- and sex-specific mortality rates match corresponding rates reported by Statistics Canada (average rates from 2014 to 2018).footnote 36 The model estimates annual excess deaths due to smoking by multiplying the stock of current smokers (and former smokers) by the difference in mortality risk between a current smoker (and former smoker) and a never smoker of the same sex and age.footnote 37
To value changes in mortality risks, estimates of the value per statistical life (VSL) are used. The VSL is an aggregated estimate of the value of small annual mortality risk changes in a population, based on estimates of individual willingness-to-pay (WTP) to reduce one's own mortality risk by a small amount. These WTP estimates are derived primarily from wage-risk studies of workers across jobs of varying risk levels. Importantly, the VSL represents the value of one “statistical life,” not the value of saving a specific individual's life. Based on the recommendations of Chestnut and DeCivita, the model uses a VSL of $7.9 million (2019 dollars).footnote 38 footnote 39
In addition to estimating the mortality impacts of smoking for current and former smokers, the model also estimates non-smoker deaths attributable to exposure to second-hand smoke (SHS). For this parameter, data on SHS-attributable mortality in 2012 from the Conference Board of Canada (2017) is used. These mortality estimates were divided by smoking prevalence in 2012 to generate SHS mortality per 1 000 smokers. The model then multiplies these mortality rates by the smoking population in each modelled year to generate estimates of SHS-attributable mortality.
Smoking-related morbidity
To estimate the economic costs associated with tobacco-related morbidity, the model relies on 2017 data on tobacco-attributable direct health care costs and short-term disability from the Canadian Substance Use Costs and Harms Scientific Working Group.footnote 40 To estimate these costs, the annual cost of short-term disability and the direct health care costs are divided by the estimated number of people aged 27 and older who smoke. Recognizing that tobacco-related illnesses generally take several years to manifest, a latency period of 10 years between smoking initiation and the onset of non-fatal health effects is assumed. Available data indicates the average age of cigarette smoking initiation is 17; therefore, health costs associated with smoking are assumed to be incurred primarily by people aged 27 and above who smoke and people who are exposed to SHS. The estimated annual morbidity cost is $2,600 (2019 dollars) for a person who smokes aged 27 and older.
Mortality and morbidity risks from vaping
Compared to the extensive information available on the health effects of smoking, there is relatively little data on the long-term health effects of using vaping products, which first became commercially available in North America in 2006. To estimate the potential adverse health effects, the model assumes that the mortality and morbidity risks associated with vaping are 20% of the mortality and morbidity impacts of cigarette use. This assumption was developed in consultation with members of an expert panel composed of five academics in tobacco control that were previously consulted on exploratory work related to a nicotine standard.footnote 41
Results of break-even analysis
The model was used to conduct a break-even analysis to determine the percentage change in the annual rate of vaping initiation from 2021 to 2050 that would be needed to generate health benefits commensurate with the estimated costs of the Regulations. It is to be noted that the vaping initiation rate includes uptake of vaping by individuals who have never vaped and those who are current smokers or former smokers. In the primary analysis, it was assumed that the Regulations will have no effect on the rate at which adults who smoke switch to vaping.
In the medium-cost scenario, the Regulations would result in a present value cost of $452 million (2019 dollars) over the period of 2021 to 2050 (assuming a 7% discount rate). To offset these costs, the Regulations would need to reduce the annual rate of vaping product initiation by 1.03% relative to the baseline initiation rate.
In the high-cost scenario, the Regulations would result in a present value cost of $1.14 billion (2019 dollars) from 2021 to 2050 (assuming a 7% discount rate). To offset these costs, the Regulations would need to reduce the annual rate of vaping product initiation by 2.56% relative to the baseline initiation rate.
| Break-even scenario | Reduction in annual vaping initiation rate required for benefits to equal costs of the Regulations | |
|---|---|---|
| Medium-cost scenario | High-cost scenario | |
| Primary analysis | 1.03% | 2.56% |
To put the analysis of public health benefits for the two break-even scenarios into perspective, Table 2 provides additional information. The numbers in this table are not a prediction of what the Regulations measures would accomplish. Rather, they illustrate the public health benefits that would be accrued in the event of a 1.03% and a 2.56% decrease in the vaping initiation rates for the medium- and high-cost scenarios. Since the estimated costs for the Regulations have been calculated, the benefits for the break-even scenario must equal or exceed these costs.
| Benefits (reduced risk) | Medium-cost scenario | High-cost scenario | ||||
|---|---|---|---|---|---|---|
| Total avoided deaths | Present value of benefits (millions of dollars) |
% of total benefits | Total avoided deaths | Present value of benefits (millions of dollars) |
% of total benefits | |
| Morbidity related to cigarette use | N/A | 34 | 7.5 | N/A | 85 | 7.5 |
| Morbidity related to vaping product use | N/A | 3 | 0.7 | N/A | 8 | 0.7 |
| Deaths attributable to cigarette use | 123 | 277 | 61.3 | 308 | 696 | 61.3 |
| Deaths attributable to vaping product use | 12 | 36 | 8.0 | 30 | 91 | 8.0 |
| Deaths attributable to second-hand smoke exposure | 45 | 102 | 22.6 | 114 | 257 | 22.6 |
| Total | 180 | 452 | 100 | 452 | 1,136 | 100 |
Sensitivity analysis
A sensitivity analysis considered the potential for the Regulations to result in a reduction in the rate at which adults who smoke switch to vaping. Specifically, the percentage change in vaping initiation needed to offset the regulatory costs under two additional scenarios was analyzed (1) assuming a 1% reduction in the annual rate at which adults who smoke switch to vaping, and (2) assuming a 10% reduction in the annual rate at which adults who smoke switch to vaping. Each scenario was evaluated for the medium- and high-cost scenarios of the Regulations. The results are presented in tables 3 and 4.
| Break-even scenario | Assumed impact on annual rate at which adults who smoke switch to vaping | Reduction in annual vaping initiation rate required for benefits to equal costs of the Regulations | |
|---|---|---|---|
| Medium-cost scenario | High-cost scenario | ||
| Primary analysis | No effect | 1.03% | 2.56% |
| Scenario 1 | 1% decrease | 1.18% | 2.72% |
| Scenario 2 | 10% decrease | 2.58% | 4.11% |
| Benefits (reduced risk) | Medium-cost scenario | High-cost scenario | ||
|---|---|---|---|---|
| Total avoided deaths | Present value of benefits (in millions of dollars) |
Total avoided deaths | Present value of benefits (in millions of dollars) |
|
| Morbidity related to cigarette use | N/A | 55 | N/A | 85 |
| Morbidity related to vaping product use | N/A | 3 | N/A | 8 |
| Deaths attributable to cigarette use | 123 | 277 | 308 | 696 |
| Deaths attributable to vaping product use | 12 | 36 | 30 | 91 |
| Deaths attributable second-hand smoke exposure | 45 | 102 | 114 | 257 |
| Total | 180 | 452 | 452 | 1,136 |
Qualitative benefits
Benefits to youth as a result of the packaging and sales prohibition
The Regulations also prohibit the packaging of vaping products in a package that displays a statement that indicates a nicotine concentration above 20 mg/mL, as well as the sale of vaping products packaged this way. These measures apply to any person, including manufacturers, importers, distributors and retailers of vaping products, that packages or sells vaping products in Canada.
Retailers have a transition period of 15 days, after the coming-into-force date, during which they will be allowed to continue to sell vaping products packaged in a manner that is contrary to these Regulations. This additional time is being provided to retailers to dispose of their inventory of non-compliant vaping products. Any additional delays in their removal at retail would result in delays in the expected benefits from efforts to prevent youth from experimenting with these products and to limit their exposure to and dependence on nicotine.
Cost-benefit statement
Summary
The Regulations are estimated to result in total incremental costs of $452.0 million PV over the 30-year period (or about $36.4 million in annualized value). The public health benefits resulting from the Regulations, including the potential benefit of protecting young persons from inducements to use vaping products, are expected to outweigh the costs of the Regulations.
- Number of years: 30 (from 2021 to 2050)
- Costing base year: 2019
- Present value base year: 2021
- Discount rate: 7%
| Impacted stakeholders | Description of cost | Base year (2021) |
Year 10 (2030) |
Year 20 (2040) |
Final year (2050) |
Total (present value) |
Annualized value |
|---|---|---|---|---|---|---|---|
| Government | Total government costs | $18,682 | $0 | $0 | $0 | $18,682 | $1,506 |
| Vaping industry | Disposal of stocks of vaping products | $58,254,497 | $0 | $0 | $0 | $58,254,497 | $4,694,520 |
| Gross profit loss | $20,557,588 | $31,265,521 | $31,265,521 | $31,265,521 | $393,737,977 | $31,729,927 | |
| All stakeholders | Total costs | $78,830,767 | $31,265,521 | $31,265,521 | $31,265,521 | $452,011,156 | $36,425,953 |
| Benefits needed to break-even the costs |
Present value of medium cost scenario assuming reduction of 10% smokers switch to vaping (2.58% reduction in vaping initiation rate) [in millions of dollars] |
|---|---|
| Reduced risk of morbidity related to cigarette use | 55 |
| Reduced risk of morbidity related to vaping product use | 3 |
| Reduced risk of death due to cigarette use | 277 |
| Reduced risk of death due to vaping | 36 |
| Reduced risk of death due to second-hand smoke exposure | 102 |
| Total | 452 |
| Impacts | Base year (2021) |
Year 10 (2030) |
Year 20 (2040) |
Final year (2050) |
Total (present value) |
Annualized value |
|---|---|---|---|---|---|---|
| Total costs | $78,830,767 | $31,265,521 | $31,265,521 | $31,265,521 | $452,011,156 | $36,425,953 |
Quantified (non-$) and qualitative impacts
Qualitative positive impact:
- Reduction in the number of youth who experiment with vaping products, which can lead to exposure to and dependence on nicotine and transition into tobacco use.
Qualitative negative impacts:
- Costs to vaping industry as a result of vapers transitioning back to smoking.
- Costs to retailers as a result of promotion restrictions that would prohibit the packaging and sale of vaping products that display a statement that indicates a nicotine concentration above 20 mg/mL.
Small business lens
Approximately 75% of vape shops, 99% of manufacturers, 80% of importers and 63% of G&C stores in the vaping industry are small businesses, under the Treasury Board of Canada Secretariat definition.footnote 42,footnote 25,footnote 32 Small businesses in British Columbia and Nova Scotia will not be impacted by the Regulations because these provinces have implemented a nicotine concentration limit of 20 mg/mL in vaping products. In Ontario, only small businesses that are specialty vape shops will be impacted by the Regulations, since Ontario only permits the sale of vaping products over 20 mg/mL nicotine in these locations. It is estimated that the vaping product market in British Columbia and Nova Scotia is about 15.5% (12.5% British Columbia and 3% Nova Scotia) of the Canadian market (in value). Due to the lack of data on the number of manufacturers and importers in British Columbia and Nova Scotia, a market share (in value) of 15.5% is used as a proxy of the number of businesses in British Columbia and Nova Scotia.
Incremental costs to all small businesses in vaping industry
Approximately 10 731 small businesses, including manufacturers (99%), importers (80%), vape shops (75%) and G&C stores (63%) across Canada, with the exception of British Columbia, Nova Scotia and Ontario (G&C stores), will assume incremental costs associated with the disposal of remaining stocks of vaping products above 20 mg/mL nicotine. It will not be possible to distribute or sell these stocks after implementation of the Regulations. Small businesses will also experience potential profit loss as a result of adult customers choosing to discontinue using vaping products altogether rather than transition to vaping products at 20 mg/mL nicotine or below after the implementation of the Regulations.
After implementation of the Regulations, the packaging of vaping products in a package with a statement that indicates a nicotine concentration above 20 mg/mL will be prohibited, as well as the sale of vaping products packaged in this way. Small manufacturers and importers might experience a loss in sales as a result of a number of adult vapers consuming vaping products above 20 mg/mL nicotine not transitioning to vaping products at 20 mg/mL nicotine or below, as well as fewer adults taking up vaping products. Consequently, those small businesses would carry incremental costs in terms of profit loss.
It is anticipated that retailers (vape shops in Canada, except those in British Columbia and Nova Scotia, and G&C stores in Canada, except those in Ontario) will also experience profit losses because they will no longer be able to sell vaping products above 20 mg/mL nicotine once these Regulations come into force.
The total costs are estimated at $290,567,797 PV over 30 years (or about $23,415,814 in annualized value). The incremental cost per impacted small business is therefore estimated at $27,078 PV over 30 years (or about $2,182 in annualized value).
In developing the Regulations, approaches that balance the minimization of regulatory burden on business with the protection of youth from inducements to use vaping products were considered. Most businesses sell vaping products with 20 mg/mL nicotine or below and the majority of adult vapers use these products. Hence, the Regulations were deemed as an appropriate option that would minimize impact on adult vapers and their access to these vaping products at retail.
Additional flexibility considered
It is estimated that the Regulations will affect 10 731 small businesses, which are composed mostly of small manufacturers, vape shops and G&C stores.
Providing additional time for small businesses to comply with the Regulations was considered. However, a longer implementation period for small businesses was deemed counter-effective in addressing the youth vaping problem. Therefore, this option was not developed.
Small business lens analysis — Costs to all impacted small businesses in vaping industry
Small business lens summary
- Number of small businesses impacted: 10 731
- Number of years: 30 (from 2021 to 2050)
- Costing base year: 2019
- Present value base year: 2021
- Discount rate: 7%
| Activity | Annualized value | Present value |
|---|---|---|
| Costs of disposing remaining stocks | $3,017,919 | $37,449,475 |
| Costs in terms of profit loss | $20,397,895 | $253,118,322 |
| Total | $23,415,814 | $290,567,797 |
| Activity | Annualized value | Present value |
|---|---|---|
| None | $0 | $0 |
| Total administrative cost |
$0 | $0 |
| Totals | Annualized value | Present value |
|---|---|---|
| Total cost (all impacted small businesses) | $23,415,814 | $290,567,797 |
| Cost per impacted small business | $2,182 | $27,078 |
One-for-one rule
There is no administrative burden on businesses that would result from the Regulations; therefore, the one-for-one rule does not apply.
Regulatory cooperation and alignment
Provincial and territorial legislation
To date, three provinces have regulated the sale of vaping products based on nicotine content. In Ontario, vaping products above 20 mg/mL nicotine can only be sold in specialty vape stores where youth do not have access. Nova Scotia and British Columbia prohibit the sale of vaping products above 20 mg/mL nicotine.
International
Article 20 of the Tobacco Products Directive (2014/40/EU) of the European Union imposes an upper limit of 20 mg/mL on the concentration of nicotine in vaping products.
Iceland, Israel, Moldova, Saudi Arabia and the United Kingdom have also adopted an upper limit of 20 mg/mL of nicotine in vaping products. South Korea has an upper limit of 10 mg/mL of nicotine in vaping products.
There is currently no restriction on the nicotine concentration of vaping products at the federal level in the United States.
The Regulations align with the limit on nicotine-containing vaping products set out in the above-mentioned jurisdictions, except for South Korea and the United States.
Strategic environmental assessment
In accordance with the Cabinet Directive on the Environmental Assessment of Policy, Plan and Program Proposals, a preliminary scan was conducted and concluded that a strategic environmental assessment is not required.
Gender-based analysis plus (GBA+)
Canadians use and experience vaping products in different ways. This is related to smoking prevalence and health disparity, current and potential vaping prevalence among different populations and the way in which nicotine is metabolized by different groups. Therefore, the Regulations could impact particular groups of Canadians differently, on the basis of age, sex, gender, mental health, substance use, socioeconomic status and other factors.
Sex differences relating to youth vaping
Data from the 2018–2019 CSTADS on past-30-day use of e-cigarettes also show no statistically significant difference in prevalence between male (21%) and female (19%) students.footnote 43
Genetic differences in nicotine metabolism
There are genetic variations in the population that affect the rate of nicotine metabolism, results in different levels of nicotine dependence.footnote 44For example, women generally metabolize nicotine faster than men, youth metabolize nicotine faster than adults, and certain groups, including some North American Indigenous populations, typically metabolize nicotine faster than others, including Black and Asian people.
Other vulnerable population groups
In general, older adolescents / young adults are more likely to try vaping products than older adults are. The 2020 CTNS data indicates that the prevalence of ever tried vaping in Canada was 35% among youth aged 15 to 19, 43% among young adults aged 20 to 24, and 13% among adults aged 25 years and older.
Vaping product use by youth can result in a dependence on nicotine and an increased risk of tobacco use. The Regulations are expected to contribute to reducing the appeal of vaping products for youth. However, the reasons youth vape are not uniform. The most commonly reported reasons for vaping among youth aged 15 to 19 who used a vaping product in the past 30 days were because they enjoyed it (27%), because they wanted to try (26%), and to reduce stress (23%).footnote 33 The Regulations may differentially impact youth depending on the reason they vape, as well as their relative dependence on nicotine.
Certain groups of Canadians have smoking rates that are considerably higher than that of the general population, including those with lower household incomes, with less education, and with mental health and substance use challenges. Strategies that reduce smoking disparity will contribute to narrowing health inequalities and to reducing the overall burden of tobacco use in Canada. Targeted actions will help to ensure no one is left behind in Canada's efforts to reach less than 5% tobacco use by 2035. In particular, the prevalence of smoking among Indigenous peoples is approximately 2 to 5 times higher than among non-Indigenous peoples in Canada, while the smoking prevalence among LGBTQ+ persons is estimated to be in the 24% to 45% range across different groups. Prevalence is also higher in certain trades: according to the 2017 Canadian Community Health Survey, close to 3 in 10 workers in the construction field or in the mining, quarrying, and oil or gas extraction fields smoked cigarettes (both 29%). This is followed closely by workers in the accommodation and food services industry, where 26% of workers in this field reported smoking.
The uptake and sustained use of vaping products by youth and non-users of tobacco products in these vulnerable groups may exacerbate the inequalities if they develop a nicotine addiction and progress to smoking. Alternatively, people who smoke in these vulnerable groups could have the potential to reduce health inequalities if they completely switch to vaping. However, there is limited data on vaping product use among these populations in Canada. Health Canada will continue to monitor the population and health inequality impacts of tobacco use. Efforts will continue by Health Canada, the Public Health Agency of Canada and Indigenous Services Canada to reach these groups with higher rates of smoking through increased resources in tobacco programs.
Implementation, compliance and enforcement, and service standards
The Regulations are made pursuant to the powers of both the TVPA and the CCPSA: the TVPA to establish a new maximum nicotine concentration of 20 mg/mL in vaping products intended for the domestic market, and the CCPSA to amend Part 2 of the VPLPR and align with this limit for products intended for the domestic market, while continuing to prohibit a nicotine concentration of 66 mg/mL or more in products intended for export. The provisions come into force on the 15th day after the day on which the Regulations are published in the Canada Gazette, Part II. Retailers have a transition period of 15 days, following the coming-into-force date, during which they will be allowed to continue to sell and dispose of vaping products that are packaged in a manner that does not meet the packaging and sale requirements set out in the Regulations.
Compliance promotion and outreach activities (including notices) aimed at informing manufacturers, importers, distributors and retailers of vaping products would take place to increase awareness of the measures set out in the Regulations and to assist parties in achieving compliance.
The Government of Canada will actively monitor compliance throughout the supply chain, including manufacturers, importers, distributors and retailers. This will occur through sampling vaping products and testing nicotine concentration using the prescribed method. If federal inspectors have reasonable grounds to believe that the Regulations have been contravened, appropriate measures will be taken under the authorities of the TVPA, which could include warning letters, compliance plans, seizures, and prosecution. Compliance and enforcement strategies will be consistent with the current overall approach to other prohibitions set out in the TVPA.
The penalties for not complying with the Regulations when they come into force are set out under Part VI of the TVPA. Every manufacturer who contravenes section 7.2 of the TVPA by manufacturing or selling a vaping product containing over 20 mg/mL nicotine, contrary to the Regulations, is guilty of an offence and liable (a) on summary of conviction to a fine not exceeding $500,000 or to imprisonment for a term not exceeding one year, or to both, or (b) on conviction on indictment to a fine not exceeding $1,000,000 or to imprisonment for a term not exceeding two years, or to both (see subsection 43(1) of the TVPA).
Every person who contravenes section 30.45 of the TVPA by packaging a vaping product in a package displaying a nicotine concentration statement indicating a concentration of nicotine above 20 mg/mL or selling a vaping product packaged this way is guilty of an offence and liable on summary conviction to a fine not exceeding $500,000 or to imprisonment for a term not exceeding two years, or to both (see section 47 of the TVPA).
Violations to section 49 of the VPLPR for exceeding the 66 mg/mL nicotine concentration limit in vaping products intended for export are, and would continue to be, assessed under the CCPSA. The penalties for not complying with the related amendments to the VPLPR when they come into force are set out in subsection 41(1) of the CCPSA. Enforcement actions under the CCPSA may include a voluntary commitment to product correction by industry, negotiation with industry for the voluntary removal of non-compliant products from the market, seizure, orders for recall or other measures, administrative monetary penalties, and possible prosecution.
The Regulations do not relate to providing a service to the public or to industry; therefore, there are no associated service standards.
Contact
Mathew Cook
Manager
Scientific Regulations Division
Tobacco Products Regulatory Office
Tobacco Control Directorate
Controlled Substances and Cannabis Branch
Health Canada
Address Locator 0301A
150 Tunney's Pasture Driveway
Ottawa, Ontario
K1A 0K9
Email: hc.pregs.sc@canada.ca