Safe Food for Canadians Regulations: SOR/2018-108
Canada Gazette, Part II, Volume 152, Number 12
Registration
May 30, 2018
CANADIAN DAIRY COMMISSION ACT
CONSUMER PACKAGING AND LABELLING ACT
CRIMINAL CODE
FEEDS ACT
FOOD AND DRUGS ACT
SEEDS ACT
HEALTH OF ANIMALS ACT
CONTROLLED DRUGS AND SUBSTANCES ACT
CUSTOMS TARIFF
SAFE FOOD FOR CANADIANS ACT
P.C. 2018-602 May 29, 2018
Her Excellency the Governor General in Council, on the recommendation of the Minister of Agriculture and Agri-Food and the Minister of Health with respect to the provisions of the annexed Regulations other than sections 391 and 408, and on the recommendation of the Minister of Justice with respect to that section 391 and of the Minister of Finance with respect to that section 408, makes the annexed Safe Food for Canadians Regulations pursuant to
- (a) section 12 of the Canadian Dairy Commission Act footnote a;
- (b) subsection 18(1) of the Consumer Packaging and Labelling Act footnote b;
- (c) subsection 462.3(2) footnote c of the Criminal Code footnote d;
- (d) subsection 5(1)footnote e of the Feeds Act footnote f;
- (e) subsection 30(1)footnote g of the Food and Drugs Act footnote h;
- (f) subsection 4(1)footnote i of the Seeds Act footnote j;
- (g) subsection 64(1)footnote k of the Health of Animals Act footnote l;
- (h) subsection 55(1)footnote m of the Controlled Drugs and Substances Act footnote n;
- (i) subsection 19(1) of the Customs Tariff footnote o; and
- (j) sections 51footnote p and 75 of the Safe Food for Canadians Actfootnote q.
TABLE OF PROVISIONS
Safe Food for Canadians Regulations
PART 1
Interpretation
1 Definitions
2 Interpretation — fresh fruits or vegetables
3 Definition prepare in Act
4 Words and expressions in documents incorporated by reference
PART 2
Trade
5 Subsection 10(1) of Act
6 Section 12 of Act
7 Subsection 13(1) of Act
8 Interprovincial trade, import and export
9 Compliance with standard
10 Use of food additives and other substances
11 Import
12 Import — fixed place of business
13 Import information
14 Import — further inspection
15 Interprovincial trade and export
16 Exception — export of non-compliant food
17 Application for export certificate
18 Non-compliant food
19 Exception — import for export
20 Exception — person who conveys
21 Personal use
22 Exception — return to Canada of exported food
23 Exception — interprovincial trade, import and export
24 Exception — in bond shipment
25 Exception — meat products
PART 3
Licences
DIVISION 1
General
26 Paragraph 20(1)(a) of Act — import
27 Paragraph 20(1)(b) of Act — import
28 Application for issuance, renewal or amendment
29 Conditions — issuance, renewal or amendment
30 Refusal — issuance, renewal or amendment
31 Establishment
32 Amendment of licence — inability to conduct activity
33 Expiry
34 Invalidity
35 Grounds for suspension
36 Suspension
37 Risk of injury to human health
38 Duration of suspension
39 Grounds for cancellation
40 Cancellation
DIVISION 2
Inspection Services — Food Animals and Meat Products
41 Inspection stations — slaughtering
42 Minimum hours of inspection — meat products
43 Inspection services outside work shifts
44 Notice
PART 4
Preventive Controls
DIVISION 1
Interpretation and Application
45 Definitions
46 Application
DIVISION 2
Biological, Chemical and Physical Hazards
47 Identification and analysis of hazards
DIVISION 3
Treatments and Processes
48 Application of scheduled process to low-acid food
DIVISION 4
Maintenance and Operation of Establishment
SUBDIVISION A
Responsibility of Operator
49 Maintenance and operation
SUBDIVISION B
Sanitation, Pest Control and Non-food Agents
50 Clean and sanitary condition
51 Animals — establishment
52 Sanitizers, agronomic inputs and non-food chemical agents
SUBDIVISION C
Conveyances and Equipment
53 Conveyances and equipment — food
54 Other conveyances and equipment
55 Equipment — restraining
SUBDIVISION D
Conditions Respecting Establishments
56 Land
57 Interior of facility or conveyance
58 Slaughtering — separate areas
59 Design, construction and maintenance — movement
60 Incompatible activities
61 Separation of food
62 Arrival of certain food at establishment
63 Lighting
64 Ventilation system
65 Temperature and humidity
66 Removal and disposal of contaminated materials and waste
67 Cleaning stations, lavatories, etc.
68 Area for inspector’s use
69 Office, lockers, etc., for inspector
70 Water — contact with food
71 Supply of water, steam and ice
SUBDIVISION E
Unloading, Loading and Storing
72 Conveyances
73 Unloading and loading
74 Storing — food
SUBDIVISION F
Competency
75 Competencies and qualifications
SUBDIVISION G
Hygiene
76 Clothing, footwear and protective coverings
77 Personal cleanliness
78 Spitting, chewing gum and other acts
79 Objects and substances — risk of contamination
80 Reporting of disease, illness, symptoms and lesions
81 Communicable disease and lesions — risk of contamination
DIVISION 5
Investigation, Notification, Complaints and Recall
82 Investigation
83 Complaints procedure
84 Recall procedure
85 Imported food
DIVISION 6
Preventive Control Plan
86 Licence holders
87 Growers or harvesters of fresh fruits or vegetables
88 Implementation
89 Content of preventive control plan
PART 5
Traceability
90 Documents
91 Production of documents
92 Labelling
PART 6
Commodity-specific Requirements
DIVISION 1
Application
93 Application — import, interprovincial trade and export
DIVISION 2
Dairy Products
94 Preparation
DIVISION 3
Eggs
95 Pasteurization
96 Import — foreign official document
97 Import — Grade C or Grade Nest Run
98 Import — ungraded eggs
99 Interprovincial trade
100 Ink
101 Trays
DIVISION 4
Processed Egg Products
102 Processing and treating eggs
103 Temperature
104 Import — foreign official document
DIVISION 5
Fish
105 Prohibition — import of mitten crab or puffer fish
106 Import of live or raw shellfish
107 Shellfish
108 Frozen fish
DIVISION 6
Fresh Fruits or Vegetables
SUBDIVISION A
Interpretation and Application
109 Definitions
110 Fresh fruits or vegetables packaged together
111 Fresh fruits or vegetables packaged with other food
SUBDIVISION B
Import
112 Whole fresh fruits or vegetables
113 Imported potatoes
114 Apples from foreign state other than United States
115 Presumption — general
116 Foreign states — onions, potatoes and apples
117 Onions, potatoes and apples from United States
118 Apples from New Zealand
119 Exception
120 In transit
121 Application for certificate
SUBDIVISION C
Trade of Fresh Fruits or Vegetables
122 Prohibition
123 Damaged or defective fresh fruits or vegetables
DIVISION 7
Meat Products and Food Animals
SUBDIVISION A
Interpretation
124 Definitions
SUBDIVISION B
Edible Meat Products
125 Identification of meat products as edible
126 Natural casings
SUBDIVISION C
Humane Treatment
127 Avoidable death — clarification
128 Avoidable suffering, injury or death
129 Hitting
130 Assessing
131 Game animals
132 Segregation and isolation
133 Overcrowding
134 Ventilation
135 Handling
136 Water and feed
SUBDIVISION D
Removal and Keeping
137 Removal
SUBDIVISION E
Ante-mortem Examination and Inspection
138 Ante-mortem examination
139 Ante-mortem inspection
140 Condemnation
SUBDIVISION F
Slaughtering and Dressing
141 Requirement before bleeding
142 Requirement after bleeding starts
143 Requirement before suspending
144 Ritual slaughter
145 Dressing
146 Blood clots, bone splinters and extraneous matter
147 Processing of blood
148 Identification and correlation
SUBDIVISION G
Post-mortem Inspection and Examination
149 Post-mortem inspection
150 Post-mortem examination
151 Inspection legend applied before refrigeration
SUBDIVISION H
Inedible Meat Products
152 Condemnation
153 Identification
154 Meat products treated as inedible
155 Inedible products area
SUBDIVISION I
Treatment
156 Contaminated meat product
157 Trichinella spp. — pork
158 Trichinella spp. — equine
159 Bovine cysticercosis
SUBDIVISION J
Post-mortem Programs
160 Application for an authorization
161 Grounds for suspension
162 Suspension
163 Risk of injury to human health
164 Suspension — duration
SUBDIVISION K
Food Animal Information Documents and Document Keeping
165 Required documents
166 Document keeping
SUBDIVISION L
Import and Export
167 Import
168 Export
PART 7
Recognition of Foreign Systems
169 Exception — shellfish
170 Application for recognition of inspection system
171 Application for recognition of system
172 Suspension of recognition — inspection system
173 Cancellation of recognition — inspection system
PART 8
Ministerial Exemptions
174 Application for exemption — test marketing or shortage in supply
175 Application for exemption — inspection legend
176 Additional conditions
177 Period of validity
178 Cancellation
PART 9
Inspection Legends
179 Definition of inspection mark in Act
180 Edible meat products — Figure 1 of Schedule 2
181 Processed egg products
182 Fish
183 Number identifying establishment
184 Authorized use
185 Advertising and sale of certain foods
PART 10
Packaging
DIVISION 1
General
186 Requirements for packages
DIVISION 2
Standard Container Sizes
187 Application
188 Table 1 of Schedule 3 — weight or volume requirements
189 Table 2 of Schedule 3 — weight requirements
190 Table 4 of Schedule 3 — volume and dimension requirements
191 Table 5 of Schedule 3 — volume and dimension requirements
192 Exception
193 Certain prepackaged fresh fruits or vegetables
DIVISION 3
Standards of Fill for Processed Fruit or Vegetable Products
194 Application
195 Frozen processed fruit or vegetable products
196 Non-frozen processed fruit or vegetable products
197 Hermetically sealed packages
PART 11
Labelling
DIVISION 1
General
SUBDIVISION A
Interpretation
198 Definitions
SUBDIVISION B
Subsection 6(1) of Act
199 False, misleading or deceptive labelling
200 Inspection — net quantity
SUBDIVISION C
Standards Prescribed for Food
201 Common names
202 Icewine
SUBDIVISION D
Information
203 Compliance with requirements of this Part
204 Use of word “classifié”
SUBDIVISION E
Official Languages
205 Prepackaged food
206 Consumer prepackaged food
207 Modifications
SUBDIVISION F
Legibility and Type Size
208 Legibility
209 Upper or lower case
210 Type size
211 Measurement of type size
DIVISION 2
Requirements Applicable to Prepackaged Food
SUBDIVISION A
Application of this Division
212 Sale, interprovincial trade or import
213 Exception — sections 214 and 217
SUBDIVISION B
Sale and Advertisement
214 Sale — prepackaged food
215 Advertising — consumer prepackaged food
216 Representations relating to net quantity
SUBDIVISION C
Label Required
217 Prepackaged food
SUBDIVISION D
Information
Prepackaged Foods
218 Prepackaged foods — label
219 Exception — common name
220 Exception — name and principal place of business
Consumer Prepackaged Foods
221 Consumer prepackaged food — declaration of net quantity
222 Place of manufacture — label or container
223 Name of importer
224 Flavouring ingredient
SUBDIVISION E
Requirement to Apply or Attach Label
225 Prepackaged food
226 Consumer prepackaged food — container
227 Principal display surface
228 Display card
SUBDIVISION F
Type Size — Specific Information
229 Consumer prepackaged food
SUBDIVISION G
Manner of Showing Declaration of Net Quantity
Legibility
230 Consumer prepackaged food
Declaration by Volume, Weight or Numerical Count
231 General requirements
Metric Units
232 Permitted units of measurement
233 Millilitres, litres, grams and kilograms
234 Number of digits
235 Quantity less than one
Metric Units and Canadian Units
236 Grouping
237 Canadian units of volume
238 Net quantity in advertisement
Individually Packaged Food Sold as One Unit and Servings
239 Individually packaged food sold as one unit
240 Prohibition — representation respecting number of servings
241 Servings
DIVISION 3
Specific Requirements for Certain Foods
SUBDIVISION A
Application of Division
242 Interprovincial trade, import and export
SUBDIVISION B
Declaration of Net Quantity
243 Exception — consumer prepackaged food
244 Declaration of net quantity
SUBDIVISION C
Location of Information
245 Food or container
SUBDIVISION D
Dairy Products
246 Prepackaged dairy products
247 Prepackaged dairy products — not consumer prepackaged
248 Consumer prepackaged dairy products
249 Consumer prepackaged cheese
250 Imported dairy products
251 Exception
252 Exported prepackaged dairy products
253 Type size
SUBDIVISION E
Eggs
254 Graded eggs
255 Size of label of graded egg
256 Imported eggs
257 Eggs to be exported
SUBDIVISION F
Processed Egg Products
258 Prepackaged processed egg products
259 Imported prepackaged processed egg products
260 Prepackaged dried egg blends
SUBDIVISION G
Fish
261 Definitions
262 Prepackaged fish
263 Prepackaged fish placed in a second container
264 Prepackaged fish — common name
265 Fish in hermetically sealed package
266 Imported prepackaged fish
267 Prepackaged whitefish
SUBDIVISION H
Fresh Fruits or Vegetables
268 Prepackaged fresh fruits or vegetables
269 Imported prepackaged fresh fruits or vegetables
270 Type size
271 Reusable plastic container
SUBDIVISION I
Processed Fruit or Vegetable Products
272 Prepackaged processed fruit or vegetable products
273 Identification name
274 Name of foreign state
SUBDIVISION J
Honey
275 Prepackaged honey
276 Graded Canadian honey
277 Imported prepackaged honey
278 Honey packaged from imported honey
279 Blend of Canadian and imported honey
SUBDIVISION K
Maple Products
280 Net quantity
281 Imported maple products
SUBDIVISION L
Edible Meat Products
282 Inspection legend — non-prepackaged edible meat products
283 Label — non-prepackaged edible meat products
284 Exception — omission or substitution of an ingredient or ingredient component
285 Definitions
286 Prepackaged edible meat products
287 Inspection legend — prepackaged edible meat products
288 Edible meat products
289 Animal species
290 Ready-to-eat edible meat products
291 Uncooked meat products
292 Prepackaged poultry carcass
293 Consumer prepackaged poultry carcass
294 Poultry carcass — not individually packaged
295 Word “ham”
296 Label of edible meat products — exception
297 Imported edible meat products
298 Imported consumer prepackaged poultry carcasses
DIVISION 4
Exceptions
299 Consumer prepackaged food
300 Declaration of net quantity
301 Raspberries or strawberries
302 Individually measured food
303 Individually packaged food sold as one unit
PART 12
Grades and Grade Names
DIVISION 1
Interpretation
304 Definitions
DIVISION 2
Grade Names
305 Definition grade name in Act
DIVISION 3
Grading
306 Mandatory grading
307 Optional grading
308 Authorized application or use
309 Imported foods — no prescribed grade name
310 Authorized reproduction
311 Advertising or sale
DIVISION 4
Packaging and Labelling
SUBDIVISION A
General
312 Labelling of grade name — consumer prepackaged food
313 Illustration of grade name
SUBDIVISION B
Eggs
314 Grade name — prepackaged eggs
315 Type size
316 Eggs — Canada A
SUBDIVISION C
Fish
317 Prepackaged fish
318 Second container
319 Type size
SUBDIVISION D
Fresh Fruits or Vegetables
320 Grade name — prepackaged fresh fruits or vegetables
321 Size designation
SUBDIVISION E
Processed Fruit or Vegetable Products
322 Size designation
SUBDIVISION F
Honey
323 Grade name — prepackaged honey
324 Colour class
SUBDIVISION G
Maple Syrup
325 Colour class
SUBDIVISION H
Livestock Carcasses
326 Prepackaged cut of beef
327 Beef — Canada AAA
328 Livestock carcass — removal of marking
329 Livestock carcass — additional marks
SUBDIVISION I
Poultry Carcasses
330 Grade name — poultry carcass
331 Packaging in same container
DIVISION 5
Conditions for Grading of Certain Foods
SUBDIVISION A
Grading of Eggs
332 Conditions for grading
333 Ungraded eggs
SUBDIVISION B
Grading of Livestock Carcasses
334 Request for grading
335 Conditions for grading
336 Adequate facilities
337 Weighing before trimming
SUBDIVISION C
Grading of Poultry Carcasses
338 Conditions for grading — dressed carcass
DIVISION 6
Grading Certificates
339 Conditions for issuance
PART 13
Organic Products
DIVISION 1
Interpretation
340 Definitions
341 Definition food commodity in Act
DIVISION 2
Packaging and Labelling
342 Packaging and labelling
DIVISION 3
Percentage of Organic Products
343 Determination of percentage of organic products
DIVISION 4
Certification
SUBDIVISION A
Organic Certification of Food Commodities
344 Application for organic certification
345 Certification
346 Requirement to provide information
SUBDIVISION B
Certification of Packaging and Labelling
347 Application for certification
348 Certification
SUBDIVISION C
Suspension and Cancellation
349 Suspension
350 Cancellation
SUBDIVISION D
General
351 Documents
352 Changes affecting certification
DIVISION 5
Labelling and Advertising
353 Expressions
354 Additional information
355 Official languages
DIVISION 6
Interprovincial Trade and Import
356 Interprovincial trade
357 Import
DIVISION 7
Product Legend
358 Definition inspection mark in Act
359 Application or use of product legend
DIVISION 8
Conformity Verification Bodies and Certification Bodies
360 Application for accreditation
361 Accreditation
362 Refusal
363 Review
364 Suspension
365 Cancellation
PART 14
Seizure and Detention
366 Detention tag
367 Prohibition — removal of detention tag
368 Notice of detention
369 Storage conditions
370 Notice of release
PART 15
Transitional Provisions
371 18-month delay
372 Fresh fruits or vegetables — 12-month delay
373 Aquaculture products — 24-month delay
374 Food commodities deemed to meet applicable requirements
375 Certificates, authorizations, exemptions, certifications and accreditations
376 Subsection 36(3) of Consumer Packaging and Labelling Regulations
377 Foreign systems deemed to be recognized
PART 16
Consequential Amendments, Repeals and Coming into Force
Consequential Amendments
Canadian Dairy Commission Act
378 EEC Aged Cheddar Cheese Export Regulations
Consumer Packaging and Labelling Act
379 Consumer Packaging and Labelling Regulations
Criminal Code
391 Regulations Excluding Certain Indictable Offences from the Definition of “Designated Offence”
Feeds Act
392 Feeds Regulations, 1983
Food and Drugs Act
393 Food and Drug Regulations
Seeds Act
401 Seeds Regulations
Health of Animals Act
403 Health of Animals Regulations
Controlled Drugs and Substances Act
407 Industrial Hemp Regulations
Customs Tariff
408 Determination of Country of Origin for the Purposes of Marking Goods (NAFTA Countries) Regulations
Repeals
Fish Inspection Act
409
Meat Inspection Act
410
Canada Agricultural Products Act
411
Coming into Force
412 S.C. 2012, c. 24
SCHEDULE 1
SCHEDULE 2
SCHEDULE 3
SCHEDULE 4
SCHEDULE 5
SCHEDULE 6
SCHEDULE 7
SCHEDULE 8
SCHEDULE 9
Safe Food for Canadians Regulations
PART 1
Interpretation
Definitions
1 The following definitions apply in these Regulations.
Act means the Safe Food for Canadians Act. (Loi)
carcass means the body of a dead animal. (carcasse)
catch-weight food means a food that because of its nature cannot normally be portioned to a predetermined fixed quantity and is, as a result, usually sold in containers of varying quantities. (aliment à poids variable)
close proximity, in respect of an item of information that is shown on a label, means immediately adjacent to the item of information and without any intervening printed, written or graphic material. (à proximité)
commercially sterile has the same meaning as in section B.27.001 of the Food and Drug Regulations. (stérilité commerciale)
common name, in respect of a food, means
- (a) the name of the food that is printed in boldface type, but not in italics, in the Standards of Identity Document or in the document entitled Common Names for Prepackaged Fish, prepared by the Agency and published on its website, as amended from time to time;
- (b) the name of the food that is printed in boldface type, but not in italics, in a provision of the Food and Drug Regulations; or
- (c) in any other case, the name by which the food is generally known or that identifies its function. (nom usuel)
Compendium means the document entitled Canadian Grade Compendium, prepared by the Agency and published on its website, as amended from time to time. (Recueil)
consumer prepackaged, in respect of a food, means packaged in a container in the manner in which the food is ordinarily sold to or used or purchased by an individual — or in which the food may reasonably be expected to be obtained by an individual — without being repackaged, to be used for non-commercial purposes. (de consommation préemballé)
container means an outer receptacle or covering that is used or to be used in connection with a food. It includes a wrapper and a confining band but does not include a conveyance or any container that is an integral part of a conveyance. (contenant)
contaminated, in respect of a food, means that the food contains any micro-organism, chemical substance, extraneous material or other substance or thing that may render the food injurious to human health or unsuitable for human consumption, including those that are not permitted under the Food and Drugs Act or those that do not comply with any limits or levels provided under that Act. (contaminé)
dairy product means milk or a food that is derived from milk, alone or combined with another food, and that contains no oil and no fat other than that of milk. (produit laitier)
dress means to dress a carcass in accordance with section 145. (habiller)
drug has the same meaning as in section 2 of the Food and Drugs Act. (drogue)
egg means an egg of a domestic chicken of the species Gallus domesticus or, in respect of a processed egg product, means that egg or an egg of a domestic turkey of the species Meleagris gallopavo. It does not include a balut. (œuf)
egg carton means a package that is capable of being closed and of containing not more than 30 eggs in separate compartments. (boîte à œufs)
eviscerate means
- (a) in respect of the carcass of a bird, other than an ostrich, rhea or emu, to remove the respiratory, digestive, reproductive and urinary systems, with or without the kidneys, and the other thoracic and abdominal organs; and
- (b) in respect of any other carcass, to remove the respiratory, digestive, reproductive and urinary systems, except the kidneys, and the other thoracic and abdominal organs. (éviscérer)
fish includes shellfish, crustaceans and other marine animals, and any of their parts, products and by-products. (poisson)
food has the same meaning as in section 2 of the Food and Drugs Act. (aliment)
food additive has the same meaning as in subsection B.01.001(1) of the Food and Drug Regulations. (additif alimentaire)
food animal means a bird or mammal, other than a marine mammal, from which an edible meat product may be derived. (animal pour alimentation humaine)
foreign state includes a WTO Member as defined in subsection 2(1) of the World Trade Organization Agreement Implementation Act. (État étranger)
game animal means a wild ruminant, pig or bird — including a ruminant, pig or bird that lives in an enclosed territory under conditions of freedom similar to those of wild animals — that is a food animal and that is hunted for commercial use under an authorization issued by a competent authority. (gibier)
hermetically sealed package means a package that, due to its design, is secure against the entry of microorganisms, including spores. (emballage hermétiquement scellé)
licence means a licence that is issued under paragraph 20(1)(a) or (b) of the Act. (licence)
livestock carcass means a beef carcass, bison carcass, ovine carcass or veal carcass. (carcasse de bétail)
maple product means a food that is obtained exclusively by the concentration of sap from trees of the genus Acer or the concentration of maple syrup. (produit de l’érable)
meat product means the carcass of a food animal, the blood of a food animal or a product or by-product of its carcass or any food that contains the blood of a food animal or a product or by-product of its carcass. It does not include
- (a) gelatin, bone meal, collagen casing, hydrolyzed animal protein, monoglycerides, diglycerides or fatty acids; or
- (b) any food that contains a meat product in an insignificant quantity, having regard to the nature of the food and of the meat product. (produit de viande)
organic product means a food commodity that has been certified as organic under subsection 345(1) or certified as organic by an entity accredited by a foreign state that is referred to in subparagraph 357(1)(a)(ii). (produit biologique)
ornamental container means a container that, except on the bottom, does not bear any advertising material, other than a trade-mark or common name, and that, because of any design appearing on its surface or because of its shape or texture, is sold both as a decorative item and as the container of a food. (contenant décoratif)
poultry carcass means the carcass of a turkey, duck, goose, guinea fowl or bird of the species Gallus domesticus. (carcasse de volaille)
prepackaged, in respect of a food, means packaged in a container in the manner in which the food is ordinarily sold to or used or purchased by a person, and includes consumer prepackaged. (préemballé)
President means the President of the Agency. (président)
principal display panel means
- (a) in the case of a consumer prepackaged food whose container is mounted on a display card, the part of the label that is applied to one or both of the following:
- (i) all or part of the principal display surface, or
- (ii) all or part of the surface of the display card that is displayed or visible under customary conditions of sale or use;
- (b) in the case of a consumer prepackaged food whose container is an ornamental container, the part of the label that is applied
- (i) to all or part of the bottom of the container,
- (ii) to all or part of the principal display surface, or
- (iii) to all or part of a tag that is attached to the container;
- (c) in the case of a consumer prepackaged food whose container is not described in paragraph (a) or (b), the part of the label that is applied to all or part of the principal display surface;
- (d) in the case of a prepackaged food other than a consumer prepackaged food, the part of the label
- (i) that is applied or attached to all or part of the surface of the container that is displayed or visible under customary conditions of sale or use, or
- (ii) if the container does not have a surface described in subparagraph (i), that is applied to any part of the container except any part that is the bottom of the container; or
- (e) in the case of a food that is not a prepackaged food, the part of the label that is applied or attached to all or part of the surface of the food that is displayed or visible under customary conditions of sale or use. (espace principal)
principal display surface, in respect of the container of a consumer prepackaged food, means
- (a) if the container has a surface that is displayed or visible under customary conditions of sale or use, the total area of that surface, excluding any surface that is the top of the container;
- (b) if the container has a lid that is the part of the container that is displayed or visible under customary conditions of sale or use, the total area of the top surface of the lid;
- (c) if the container does not have a particular surface that is displayed or visible under customary conditions of sale or use, 40% of the total surface area of the container, excluding any surface area that is its top and bottom, if it is possible for that 40% to be displayed or visible under customary conditions of sale or use;
- (d) if the container is a bag with surfaces of equal dimensions, the total area of one of the surfaces;
- (e) if the container is a bag with surfaces of different dimensions, the total area of one of the largest surfaces;
- (f) despite paragraphs (a) to (e), if the container does not have a surface that is displayed or visible under customary conditions of sale or use to which a label can be applied, the total area of one side of a tag that is attached to the container;
- (g) despite paragraphs (a) to (e), if the container contains wine that is exposed for sale, any part of the surface of the container, excluding its top and bottom, that can be seen without having to turn the container; and
- (h) if the container is a wrapper or confining band that is so narrow in relation to the size of the food that it cannot reasonably be considered to have any surface that is displayed or visible under customary conditions of sale or use, the total area of one side of a tag that is attached to the container. (principale surface exposée)
processed egg product means a food for which a standard is set out in Volume 2 of the Standards of Identity Document. (produit d’œufs transformés)
processed fruit or vegetable product means a food
- (a) for which a standard is set out in Volume 4 of the Standards of Identity Document;
- (b) for which a grade is set out in Volume 3 of the Compendium;
- (c) that is set out in column 1 of Table 3 of Schedule 3 in items 2 to 11 or in column 1 of Table 4, 5 or 6 of that Schedule; or
- (d) to which Division 3 of Part 10 applies. (produit de fruits ou de légumes transformés)
ready-to-eat, in respect of an edible meat product, means that it has been subjected to a treatment or process that is sufficient to inactivate vegetative pathogenic micro-organisms or their toxins and control spores of food-borne pathogenic bacteria so that the meat product does not require further preparing before consumption except washing or thawing or exposing it to sufficient heat to warm it without cooking it. (prêt à manger)
refrigerated, in respect of a food, means that it is kept at a temperature of 4°C or less, without being frozen. (réfrigéré)
sanitary condition means a condition that does not present a risk of contamination of a food. (conditions hygiéniques)
shellfish means a bivalve mollusc of the class Bivalvia or a carnivorous marine mollusc of the class Gastropoda, or any product that is derived from one of those molluscs. (mollusque)
Standards of Identity Document means the document entitled Canadian Standards of Identity, prepared by the Agency and published on its website, as amended from time to time. (Document sur les normes d’identité)
tray, in respect of eggs, means a package, other than an egg carton, that is capable of containing not more than 30 eggs in separate compartments. (plateau)
wine means an alcoholic beverage that meets the standard set out in section B.02.100 of the Food and Drug Regulations. (vin)
Interpretation — fresh fruits or vegetables
2 (1) For the purposes of any provision of these Regulations that refers to “fresh fruits or vegetables”, other than section 122, any fresh plant or any fresh edible fungus, or any part of such a plant or fungus, that is a food is considered to be a fresh fruit or vegetable.
Exception — subparagraph 11(2)(c)(i)
(2) For the purposes of any provision of these Regulations that refers to “fresh fruits or vegetables”, a food described in subparagraph 11(2)(c)(i) is not considered to be a fresh fruit or vegetable.
Definition prepare in Act
3 For the purposes of the definition prepare in section 2 of the Act, the growing and harvesting of any fresh fruits or vegetables are prescribed activities.
Words and expressions in documents incorporated by reference
4 For the purposes of interpreting any document prepared by the Agency that is incorporated by reference into these Regulations, words and expressions that are used but not defined in that document have the same meaning as in these Regulations.
PART 2
Trade
Subsection 10(1) of Act
5 (1) For the purposes of subsection 10(1) of the Act, any food commodity is a prescribed food commodity that a person is prohibited to send or convey from one province to another — or to import or export — unless the person does so in accordance with these Regulations.
Subsection 10(2) of Act
(2) For the purposes of subsection 10(2) of the Act, any food — other than a food referred to in any of paragraphs 11(2)(a) to (c) of these Regulations — is a prescribed food commodity that a person is prohibited to import unless the person is authorized to do so by a licence.
Subsection 10(3) of Act
(3) For the purposes of subsection 10(3) of the Act, any food commodity is a prescribed food commodity that a person is prohibited to send or convey from one province to another — or to import or export — unless it meets the requirements of these Regulations.
Section 12 of Act
6 For the purposes of section 12 of the Act, any food commodity is a prescribed food commodity that a person is prohibited to have in their possession for the purpose of sending or conveying from one province to another — or for the purpose of exporting — unless it meets the requirements of these Regulations.
Subsection 13(1) of Act
7 (1) For the purposes of subsection 13(1) of the Act, any food commodity is a prescribed food commodity that is to be exported or sent or conveyed from one province to another, and any of the following activities is a prescribed activity that a person is prohibited to conduct in respect of that prescribed food commodity except in accordance with these Regulations:
- (a) manufacturing, preparing, storing, packaging and labelling; and
- (b) if the food commodity is an organic product, in addition to the activities set out in paragraph (a), advertising and conveying.
Subsection 13(2) of Act
(2) For the purposes of subsection 13(2) of the Act, any food — other than a food referred to in paragraph 11(2)(a) or (b) of these Regulations — and any food animal is a prescribed food commodity that is to be exported or sent or conveyed from one province to another, and any of the following activities is a prescribed activity that a person is prohibited to conduct in respect of that prescribed food commodity unless the person is authorized to conduct that activity by a licence:
- (a) in the case of a food,
- (i) manufacturing, processing, treating, preserving, grading, packaging and labelling, other than
- (A) the packaging and labelling of fresh fruits or vegetables in the field by the person who grows or harvests them if they are to be sent or conveyed from one province to another to be subsequently manufactured, processed, treated, preserved or graded by a licence holder,
- (B) the packaging and labelling of a food referred to in subparagraph 11(2)(c)(i) if, at the time that the food is exported or is sent or conveyed from one province to another, it is not a consumer prepackaged food and it has a label applied or attached to it, or accompanying it, that bears the expression “For Further Preparation Only” or “pour conditionnement ultérieur seulement”, and
- (C) the grading of a livestock carcass and a poultry carcass, and
- (ii) if the food is an edible meat product, in addition to the activities set out in subparagraph (i), storing and handling in its imported condition for the purpose of the exercise of an inspector’s powers under the Act; and
- (i) manufacturing, processing, treating, preserving, grading, packaging and labelling, other than
- (b) in the case of a food animal, slaughtering.
Interprovincial trade, import and export
8 (1) Any food that is sent or conveyed from one province to another or that is imported or exported
- (a) must not be contaminated;
- (b) must be edible;
- (c) must not consist in whole or in part of any filthy, putrid, disgusting, rotten, decomposed or diseased animal or vegetable substance; and
- (d) must have been manufactured, prepared, stored, packaged and labelled under sanitary conditions.
Prohibition — mixing of contaminated food
(2) It is prohibited for a person to mix a contaminated food with other food so that it meets the requirements of subsection (1), unless the person is authorized to do so by the Minister under subsection (3).
Ministerial authorization
(3) The Minister may authorize a person to mix a contaminated food with other food if the Minister is of the opinion that no risk of injury to human health will result.
Compliance with standard
9 (1) Any food that is sent or conveyed from one province to another or that is imported or exported and for which a standard is set out in the Standards of Identity Document must comply with that standard.
Food likely to be mistaken
(2) Any food that is sent or conveyed from one province to another or that is imported or exported and that is likely to be mistaken for a food for which a standard is set out in the Standards of Identity Document must comply with that standard.
Use of food additives and other substances
10 Any person who manufactures, processes, treats or preserves a food that has been imported or that is to be exported or sent or conveyed from one province to another may use, in or on the food, a food additive or other substance unless the use of that food additive or other substance is not permitted by these Regulations or under the Food and Drugs Act or the use does not comply with any limits or levels provided by these Regulations or under that Act.
Import
11 (1) Any food that is imported must have been manufactured, prepared, stored, packaged and labelled in a manner and under conditions that provide at least the same level of protection as that provided by sections 47 to 81.
Exception
(2) Subsection (1) does not apply in respect of
- (a) a food additive;
- (b) a beverage that contains more than 0.5% absolute ethyl alcohol by volume; or
- (c) a food that is set out in Schedule 1 and that
- (i) is unprocessed and is intended to be manufactured, processed or treated for use as a grain, oil, pulse, sugar or beverage,
- (ii) has a label applied or attached to it, or accompanying it, that bears the expression “For Further Preparation Only” or “pour conditionnement ultérieur seulement”, and
- (iii) is not a consumer prepackaged food.
Import — fixed place of business
12 (1) Any person who imports a food, other than a food referred to in any of paragraphs 11(2)(a) to (c), and who does not have, in Canada, a fixed place of business from which they carry on business related to the food must,
- (a) have a fixed place of business from which they carry on business related to the food in a foreign state that
- (i) has an inspection system that has been recognized under Part 7, if the imported food is a meat product or live or raw shellfish, or
- (ii) has a food safety system that has been determined by the Minister under subsection (2) to provide at least the same level of protection in relation to that food as that provided by the provisions of the Act and these Regulations, if the imported food is not a meat product or live or raw shellfish; and
- (b) send or convey the food directly to Canada from a foreign state that has an inspection system described in subparagraph (a)(i) or a food safety system described in subparagraph (a)(ii).
Food safety system — Minister’s determination
(2) The Minister must determine whether a foreign state’s food safety system provides at least the same level of protection in relation to an imported food as that provided by the provisions of the Act and these Regulations by taking into account the following:
- (a) any applicable legislative framework, controls and procedures;
- (b) the organizational structure of the authority that is responsible for the system;
- (c) the implementation of the system;
- (d) the resources that support the objectives of the system; and
- (e) any other relevant information.
Exception — certain shellfish
(3) For the purposes of paragraph (1)(a), the reference to “shellfish” does not include the adductor muscles of scallops or the meat of geoducks.
In transit
(4) For the purposes of paragraph (1)(b), if the food passes only in transit through a foreign state, the person is not considered to have sent or conveyed the food directly to Canada from that foreign state.
Import information
13 (1) Any person who imports a food must provide to the Minister, in a form approved by the President, the following information:
- (a) their name and address and, if they hold a licence to import, the number of that licence;
- (b) the name and address of the person from whom the food is received;
- (c) the name of the foreign state of origin;
- (d) the address of the first destination of the food in Canada;
- (e) a description of the food, including its common name and quantity;
- (f) any information relating to the safety of the food that the Minister believes is required in order to respond to a risk of injury to human health; and
- (g) in the case of live or raw shellfish other than the adductor muscles of scallops or the meat of geoducks, with respect to the establishment at which the shellfish was last manufactured, prepared, stored, packaged or labelled prior to its importation, the establishment’s registration number, or another identification number for the establishment, that is provided by the foreign state.
Provision of import information
(2) The information referred to in subsection (1), and any documents required by sections 96 and 104 and paragraph 167(d), must be provided before or at the time of the import.
Exception
(3) Despite subsection (2), in the case of a food other than a meat product, the Minister may authorize the person who imports the food, at their written request, to provide the information after the time of import, at the time specified by the Minister.
Meat products
(4) For the purposes of subsection (3), the foods set out in paragraphs 25(a) and (b) are not considered to be meat products.
Import — further inspection
14 (1) If, during an inspection that is conducted at the time of the import, the inspector determines that a further inspection is required,
- (a) in the case of an edible meat product,
- (i) the meat product must be immediately delivered, by the licence holder who imports it, to an establishment where it must be stored and handled in its imported condition by a licence holder and must be kept in that establishment until the further inspection is completed, and
- (ii) the licence holder who imports the meat product must provide the address of the establishment referred to in subparagraph (i) to the inspector if it is different from the address referred to in paragraph 13(1)(d); and
- (b) in the case of a food other than an edible meat product, the food must be kept, by the person who imports it, at the address referred to in paragraph 13(1)(d) until the further inspection is completed.
Meat products
(2) For the purposes of subsection (1), the foods set out in paragraphs 25(a) and (b) are not considered to be meat products.
Interprovincial trade and export
15 (1) Any food that is sent or conveyed from one province to another or that is exported must meet the following requirements:
- (a) if the food is manufactured, processed, treated, preserved, graded, packaged or labelled in Canada, that activity must be conducted by a licence holder in accordance with the provisions of the Act and these Regulations, unless that activity is
- (i) the packaging and labelling of fresh fruits or vegetables in the field by the person who grows or harvests them if they are to be sent or conveyed from one province to another to be subsequently manufactured, processed, treated, preserved or graded by a licence holder,
- (ii) the packaging and labelling of a food that is set out in Schedule 1 and that is unprocessed and intended to be manufactured, processed or treated for use as a grain, oil, pulse, sugar or beverage, if, at the time that the food is sent or conveyed from one province to another or exported, it is not a consumer prepackaged food and it has a label applied or attached to it, or accompanying it, that bears the expression “For Further Preparation Only” or “pour conditionnement ultérieur seulement”, or
- (iii) the grading of a livestock carcass or a poultry carcass;
- (b) if the food, other than a food referred to in paragraph 11(2)(c), has been imported, it must have been imported by a licence holder in accordance with the provisions of the Act and these Regulations; and
- (c) if the food is a meat product and if
- (i) any meat product that it contains was manufactured, processed, treated, preserved, packaged or labelled in Canada, that activity must have been conducted by a licence holder in accordance with the provisions of the Act and these Regulations,
- (ii) any meat product that it contains has been derived from a livestock carcass or a poultry carcass that has been graded in Canada, it must have been graded by a grader in accordance with these Regulations,
- (iii) any meat product that it contains has been imported, that meat product must have been imported by a licence holder in accordance with the provisions of the Act and these Regulations, and
- (iv) any meat that it contains is derived from food animals that were slaughtered in Canada, the food animals must have been slaughtered by a licence holder in accordance with the provisions of the Act and these Regulations.
Exception
(2) Subsection (1) does not apply in respect of the foods referred to in paragraphs 11(2)(a) and (b).
Exception — export of non-compliant food
16 (1) Any person may export a food that does not meet the requirements of these Regulations, other than a requirement of paragraph 8(1)(c) or (d) or subsection 15(1), if a label applied or attached to the food bears the word “Export” or “exportation” and
- (a) if the foreign state to which the food is exported has a different requirement on the same matter as the unmet requirement, the person prepares a document that substantiates that the foreign state’s requirement has been met; or
- (b) if the foreign state to which the food is exported has no requirement on the same matter as the unmet requirement,
- (i) the unmet requirement must be a requirement set out in any of subsection 9(1), sections 10, 188 to 192, 195, 197, 201, 210, 244 to 249, 253 and 255, subsection 257(2), paragraphs 258(c) and (d), sections 262, 264, 265, 267, 268, 272, 273, 275 and 280, paragraph 286(a), sections 288, 292 to 295, 306 to 308, 312, 313, 316, 322, 324 to 327, 329 and 331, and
- (ii) the person prepares a document that sets out the specifications for the unmet requirement as stipulated by the person in the foreign state for whom the exported food is intended.
Retention period — documents
(2) The documents referred to in paragraph (1)(a) and subparagraph (1)(b)(ii) must be kept for two years after the day on which the food is exported.
Additional requirements — meat products
(3) In the case of the export of a meat product under subsection (1), the requirements of paragraphs 168(1)(a) and (b) must also be met.
Application for export certificate
17 (1) An application for the issuance of a certificate or other document referred to in section 48 of the Act must be made to the Minister in a form approved by the President.
Conditions for issuance
(2) The Minister may issue a certificate or other document referred to in section 48 of the Act in respect of a food commodity that is intended for commercial use if the applicant holds a licence to export the food commodity and
- (a) in the case of a food, the manufacturing, preparing, storing, packaging and labelling of the food meets the applicable requirements of Part 4;
- (b) in the case of a food commodity referred to in paragraph (b) of the definition food commodity in section 2 of the Act, other than an animal, for which a competent authority of a foreign state requires a certificate or other document referred to in section 48 of the Act for its import into that foreign state for the purpose of human consumption, the manufacturing, preparing, storing, packaging and labelling of the food commodity meets the applicable requirements of Part 4 — other than Division 3 — as if it were a food; or
- (c) in the case of a food commodity referred to in subsection (3), the manufacturing, preparing, storing, packaging and labelling of the food commodity meets the applicable requirements of Part 4 — other than Division 3 — as if it were a food.
Definition of food commodity in Act
(3) For the purposes of paragraph (c) of the definition food commodity in section 2 of the Act, any commodity that is derived from an animal or plant, or any of its parts, is a prescribed food commodity if
- (a) that commodity is not included in paragraph (a) or (b) of that definition; and
- (b) a competent authority of a foreign state requires a certificate or other document referred to in section 48 of the Act in order for that commodity to be imported into that foreign state for the purpose of human consumption.
Exemption
(4) The prescribed food commodity referred to in subsection (3) is exempted from the application of any provision of the Act and these Regulations that is not necessary to give effect to this section. For greater certainty, the exemption does not include section 6 of the Act.
Inspection before export
(5) The Minister may require that an inspection be conducted of any food commodity in respect of which a person has applied for a certificate or other document referred to in section 48 of the Act, for the purpose of deciding whether to issue the certificate or other document.
Inspection — accessibility
(6) If an inspection of a food commodity is required, the applicant must make the food commodity readily accessible to an inspector at the time of inspection.
Non-compliant food
18 (1) Any person may send or convey from one province to another or import a food that does not meet the requirements of the Act or these Regulations — other than section 189 as that section relates to fresh fruits or vegetables, processed fruit or vegetable products or honey, sections 190 to 193, section 306 as that section relates to fresh fruits or vegetables or processed fruit or vegetable products and Volume 4 of the Standards of Identity Document — if
- (a) a label that bears the expression “For Further Preparation Only” or “pour conditionnement ultérieur seulement” is applied or attached to the food or accompanies it;
- (b) subject to subsection 8(2), the food is manufactured, processed, treated, preserved, graded, packaged or labelled so that it meets the requirements that are set out in the Act and these Regulations within
- (i) three months after the day on which the food is sent or conveyed from one province to another or is imported, or
- (ii) any longer period that is specified by the Minister at the person’s written request; and
- (c) in the case of import, the food is not a meat product.
Exception
(2) Paragraph (1)(a) does not apply in respect of
- (a) a beef carcass, or a carcass side, hind quarter, front quarter, primal cut or sub-primal cut of a beef carcass, that meets the requirements that are set out in paragraph 306(2)(f);
- (b) a processed fruit or vegetable product that is labelled under paragraph 306(3)(a); or
- (c) honey that is labelled under paragraph 306(3)(b).
Licence holder
(3) The activities referred to in paragraph (1)(b), other than the grading of a livestock carcass or a poultry carcass, must be conducted by a licence holder.
Exception — import for export
19 (1) Any person may import a food that does not meet the requirements of the Act or these Regulations — other than section 189 as that section relates to fresh fruits or vegetables, processed fruit or vegetable products or honey, sections 190 to 193, section 306 as that section relates to fresh fruits or vegetables or processed fruit or vegetable products, Part 13 and Volume 4 of the Standards of Identity Document — if
- (a) a label that bears the expression “Imported for Export” or “importé pour l’exportation” is applied or attached to the food or accompanies it; and
- (b) the food is intended to be manufactured, processed, treated, preserved, graded, packaged or labelled for the purpose of exporting it.
Licence holder
(2) The activities referred to in paragraph (1)(b), other than the grading of a livestock carcass or a poultry carcass, must be conducted by a licence holder.
Exception — person who conveys
20 (1) Subject to subsection (2), the provisions of the Act and these Regulations do not apply to any person who conveys a food commodity if their sole concern, in respect of the food commodity, is its conveyance.
Exception
(2) Sections 122 and 123 and subsection 359(3), and any provisions of the Act and these Regulations that are necessary to give effect to them, apply to the person referred to in subsection (1).
Personal use
21 For the purposes of section 19 of the Act, the sending or conveying from one province to another, or the import or export, of a food is considered to be an activity carried out solely for personal use if the food is not intended for commercial use and if
- (a) it is sent or conveyed, imported or exported by an individual, otherwise than in the course of business, and is part of a shipment of food in a quantity that is not more than the quantity set out in the document entitled Maximum Quantity Limits for Personal Use Exemption, prepared by the Agency and published on its website, as amended from time to time; or
- (b) it is imported or exported and is part of the personal effects of an immigrant or emigrant.
Exception — return to Canada of exported food
22 (1) The requirements of the Act and these Regulations in relation to import do not apply in respect of a food that is imported after having been exported from Canada if the food is in its exported condition and if
- (a) in the case of a food other than an edible meat product, the food is sent back to
- (i) the person who exported it from Canada, if that person holds a licence to export, or
- (ii) the person who was last in possession of it before its export, from among the persons who manufactured, processed, treated, preserved, graded, packaged or labelled it; and
- (b) in the case of an edible meat product, the import is authorized by an inspector and the meat product is immediately delivered to an establishment where it is stored and handled in its imported condition by a licence holder and kept in that establishment until an inspector has completed an inspection of the product.
Meat products
(2) For the purposes of subsection (1), the foods set out in paragraphs 25(a) and (b) are not considered to be meat products.
Exception — interprovincial trade, import and export
23 (1) The provisions of the Act and these Regulations do not apply in respect of a food that is sent or conveyed from one province to another or that is imported or exported if
- (a) the food is carried on a conveyance for use by the crew or passengers of that conveyance;
- (b) the food is intended and used for analysis, evaluation, research or a Canadian or international food exhibition and is part of a shipment that weighs 100 kg or less or, in the case of eggs, is part of a shipment of five or fewer cases that are each intended to contain 30 dozen eggs;
- (c) the food is not intended or sold for use as food and a label that indicates its intended use and bears the expression “Not for Use as Human Food” or “ne peut servir à l’alimentation humaine” is applied or attached to the food or accompanies it;
- (d) in the case of a food that is imported, the food
- (i) is imported from the United States into the Akwesasne Reserve for use by an individual who has established permanent residence on that Reserve, or
- (ii) is part of an in bond shipment that is sent or conveyed from a foreign state to a cruise ship or military ship in Canada for use by the crew or passengers; or
- (e) in the case of a food that is sent or conveyed from one province to another, the food is sent or conveyed from one federal penitentiary to another.
In transit
(2) For the purposes of subparagraph (1)(d)(i), if the food is part of an in bond shipment that passes only in transit through the United States, the food is not considered to have been imported from the United States.
Exception — in bond shipment
24 The provisions of the Act and these Regulations do not apply in respect of a food that is part of an in bond shipment that is sent or conveyed from one foreign state to another if
- (a) the food is manufactured or prepared in a foreign state; and
- (b) the food passes only in transit through Canada.
Exception — meat products
25 Subparagraph 7(2)(a)(ii), subsection 28(2), paragraph 29(1)(d), subsection 31(2), section 42, paragraph 46(1)(b) and sections 69, 167, 168 and 296 do not apply in respect of
- (a) meat products — other than those set out in column 1 of Part A of Table 2 to Volume 7 of the Standards of Identity Document — that are a mixture of a ready-to-eat meat product and a food other than a meat product, if
- (i) the ready-to-eat meat product that is contained in the mixture was manufactured, processed, treated, preserved, packaged or labelled in Canada by a licence holder in accordance with the provisions of the Act and these Regulations,
- (ii) the ready-to-eat meat product that is contained in the mixture is derived from a livestock carcass or poultry carcass that has been graded in Canada by a grader in accordance with these Regulations,
- (iii) the ready-to-eat meat product that is contained in the mixture has been imported by a licence holder in accordance with the provisions of the Act and these Regulations, or
- (iv) the mixture is imported and
- (A) the foreign state in which the mixture is manufactured, prepared, stored, packaged or labelled, as the case may be, has, at the time the activity is conducted, an inspection system for meat products that is recognized under Part 7,
- (B) the foreign state from which the mixture is imported has, at the time of the import, an inspection system for meat products that is recognized under Part 7,
- (C) the establishment where the food animal from which the ready-to-eat meat product that is contained in the mixture is derived was slaughtered, and any establishment where that meat product was manufactured, processed, treated, preserved, handled, tested, graded, coded, stored, packaged or labelled, have, at the time that the activity is conducted and at the time of the import, a system for manufacturing, processing, treating, preserving, handling, testing, grading, coding, slaughtering, storing, packaging or labelling, as the case may be, that is recognized under Part 7, and
- (D) the holder of the licence to import provides an inspector with an official document issued by the foreign state, in a form approved by the President, that states that the ready-to-eat meat product that is contained in the mixture meets the requirements that are set out in the Act and these Regulations; and
- (b) broth, lard, leaf lard, tallow or other rendered fat, suet, shortening, flavour or extract if
- (i) the meat product from which the broth, lard, leaf lard, tallow or other rendered fat, suet, shortening, flavour or extract is derived was manufactured, processed, treated, preserved, packaged or labelled in Canada by a licence holder in accordance with the provisions of the Act and these Regulations,
- (ii) the meat product from which the broth, lard, leaf lard, tallow or other rendered fat, suet, shortening, flavour or extract is derived is a livestock carcass or a poultry carcass that has been graded in Canada by a grader in accordance with these Regulations,
- (iii) the meat product from which the broth, lard, leaf lard, tallow or other rendered fat, suet, shortening, flavour or extract is derived has been imported by a licence holder in accordance with the provisions of the Act and these Regulations, or
- (iv) the broth, lard, leaf lard, tallow or other rendered fat, suet, shortening, flavour or extract is imported and
- (A) the foreign state in which the broth, lard, leaf lard, tallow or other rendered fat, suet, shortening, flavour or extract is manufactured, prepared, stored, packaged or labelled, as the case may be, has, at the time the activity is conducted, an inspection system for meat products that is recognized under Part 7,
- (B) the foreign state from which the broth, lard, leaf lard, tallow or other rendered fat, suet, shortening, flavour or extract is imported has, at the time of the import, an inspection system for meat products that is recognized under Part 7,
- (C) the establishment where the food animal from which the meat product from which the broth, lard, leaf lard, tallow or other rendered fat, suet, shortening, flavour or extract is derived was slaughtered, and any establishment where the meat product was manufactured, processed, treated, preserved, handled, tested, graded, coded, stored, packaged or labelled, have, at the time that the activity is conducted and at the time of the import, a system for manufacturing, processing, treating, preserving, handling, testing, grading, coding, slaughtering, storing, packaging or labelling, as the case may be, that is recognized under Part 7, and
- (D) the holder of the licence to import keeps a document that substantiates that the conditions set out in clauses (A) to (C) are met.
PART 3
Licences
DIVISION 1
General
Paragraph 20(1)(a) of Act — import
26 (1) For the purpose of issuing a licence to import under paragraph 20(1)(a) of the Act, any food is a prescribed food commodity.
Paragraph 20(1)(a) of Act — export
(2) For the purpose of issuing a licence to export under paragraph 20(1)(a) of the Act, any of the following is a prescribed food commodity:
- (a) any food;
- (b) any food commodity referred to in paragraph 17(2)(b); and
- (c) any food commodity referred to in subsection 17(3).
Paragraph 20(1)(b) of Act — import
27 (1) For the purpose of issuing a licence under paragraph 20(1)(b) of the Act, any food is a prescribed food commodity that has been imported, and the storing and handling of a food in its imported condition for the purpose of the exercise of an inspector’s powers under the Act are prescribed activities in respect of that prescribed food commodity.
Paragraph 20(1)(b) of Act — interprovincial trade
(2) For the purpose of issuing a licence under paragraph 20(1)(b) of the Act, any food and any food animal are the prescribed food commodities that are to be sent or conveyed from one province to another, and the following activities are prescribed activities in respect of those prescribed food commodities:
- (a) in the case of a food, manufacturing, processing, treating, preserving, grading, storing, packaging and labelling; and
- (b) in the case of a food animal, slaughtering.
Paragraph 20(1)(b) of Act — export
(3) For the purpose of issuing a licence under paragraph 20(1)(b) of the Act, any food, any food animal, any food commodity referred to in paragraph 17(2)(b) and any food commodity referred to in subsection 17(3) are prescribed food commodities that are to be exported, and the following activities are prescribed activities in respect of those prescribed food commodities:
- (a) in the case of a food, a food commodity referred to in paragraph 17(2)(b) and a food commodity referred to in subsection 17(3), manufacturing, processing, treating, preserving, grading, storing, packaging and labelling; and
- (b) in the case of a food animal, slaughtering.
Application for issuance, renewal or amendment
28 (1) An application for the issuance, renewal or amendment of a licence must be made to the Minister in a form approved by the President.
Application — food animals and meat products
(2) An application for the issuance, renewal or amendment of a licence to slaughter a food animal, to manufacture, process, treat, preserve, grade, package or label a meat product or to store and handle an edible meat product in its imported condition must include at least one proposed work shift for each establishment where the activity is conducted.
Work shift
(3) A work shift must be
- (a) in the case of slaughtering, a work shift during which no inspection station referred to in subsection 41(1) is operated for more than 7.5 hours in one day and 37.5 hours in one work week, excluding meal times; and
- (b) in the case of manufacturing, processing, treating, preserving, grading, packaging or labelling a meat product or storing and handling an edible meat product in its imported condition, a work shift during which those activities are conducted
- (i) for not more than 7.5 hours in one day and 37.5 hours in one work week, excluding meal times, or
- (ii) between 6:00 a.m. and 6:00 p.m.
Conditions — issuance, renewal or amendment
29 (1) The Minister may issue, renew or amend a licence if
- (a) in the case of an application for the issuance of a licence, the applicant is not in default of payment of any fee related to the Act that is fixed under the Canadian Food Inspection Agency Act;
- (b) the applicant, whether or not they are a licence holder conducting the activity in respect of which the application for the issuance, renewal or amendment of the licence is made,
- (i) in the case of a licence in respect of a food, meets the applicable requirements of Part 4, and
- (ii) in the case of a licence in respect of a food commodity referred to in paragraph 17(2)(b) or subsection 17(3), meets the applicable requirements of Part 4 — other than Division 3 — as if the food commodity were a food;
- (c) in the case of an application for the issuance, renewal or amendment of a licence to import, the applicant carries on business related to the food in respect of which the application is made from a fixed place of business that is in
- (i) Canada, or
- (ii) a foreign state that has an inspection system described in subparagraph 12(1)(a)(i) or a food safety system described in subparagraph 12(1)(a)(ii);
- (d) in the case of an application for the issuance, renewal or amendment of a licence to slaughter a food animal, to manufacture, process, treat, preserve, grade, package or label a meat product or to store and handle an edible meat product in its imported condition, a work shift has been approved by the President for each establishment where the activity is conducted;
- (e) the information submitted in the application is complete, truthful and not misleading; and
- (f) the Minister is of the opinion, on the basis of the information that was made available to him or her, that the conduct of the activity in respect of which the application for the issuance, renewal or amendment of the licence is made does not present a risk of injury to human health.
Renewal of suspended licence
(2) Despite paragraph (1)(b), the Minister may renew a suspended licence if the requirements of subsection (1), other than any requirements whose contravention forms the grounds of the suspension, are met. However, the suspension of the licence remains in effect.
Refusal — issuance, renewal or amendment
30 The Minister may refuse to issue, renew or amend a licence if
- (a) in the five years before the day on which the application is made, the applicant or any of their directors or officers
- (i) have had a licence suspended or cancelled, or
- (ii) have been convicted of an offence under the Act or the Food and Drugs Act; or
- (b) in the case of an application for the renewal or amendment of a licence, the applicant is in default of payment of any fee related to the licence that is fixed under the Canadian Food Inspection Agency Act.
Establishment
31 (1) A licence holder must conduct the activities that are identified in their licence, other than importing and exporting, in the establishment that is identified in the licence for the activities.
Work shift
(2) Subject to subsection (3) and unless otherwise authorized by an inspector, the activities that are conducted in respect of a food animal or meat product must be conducted during a work shift approved by the President and during which inspection services are provided in accordance with Division 2.
Exception — ante-mortem examination
(3) In the case of the slaughtering of a food animal, the ante-mortem examination may be conducted outside a work shift.
Amendment of licence — inability to conduct activity
32 (1) If a licence holder is unable to conduct an activity that is identified in their licence in one of the establishments that are identified in the licence, the Minister may amend the licence to remove the authorization to conduct that activity in that establishment.
Written notice
(2) The Minister must notify the licence holder in writing of the amendment and the date on which it takes effect.
Expiry
33 (1) A licence expires two years after the date of issuance or renewal that is specified in it, unless the licence is cancelled before that date.
Expiry — amendment
(2) If the Minister amends a licence, its expiry date remains unchanged.
Invalidity
34 A licence becomes invalid if the licence holder surrenders the licence to the Minister and it is not subject to a cancellation procedure.
Grounds for suspension
35 The Minister may suspend a licence if
- (a) the licence holder does not comply with any provision of the Act, other than section 15, or with any provision of these Regulations, the Food and Drugs Act or the Food and Drug Regulations;
- (b) the licence holder is in default of payment of any fee related to the licence that is fixed under the Canadian Food Inspection Agency Act; or
- (c) the Minister is of the opinion that a risk of injury to human health may result if the licence holder continues to conduct an activity that is identified in the licence.
Suspension
36 (1) The Minister must not suspend a licence unless the licence holder
- (a) was provided with a written report that sets out the grounds for the suspension and the period within which corrective action must be taken in order to avoid the suspension; and
- (b) failed to take corrective action within that period.
Written notice
(2) The Minister must notify the licence holder in writing of the suspension and the date on which it takes effect.
Risk of injury to human health
37 (1) Despite section 36, if the Minister is of the opinion that a risk of injury to human health may result if the licence holder continues to conduct an activity that is identified in the licence, the Minister may suspend the licence immediately after the licence holder is provided with a written report that sets out the grounds for the suspension.
Written notice
(2) The Minister must notify the licence holder in writing that their licence is suspended and that the suspension takes effect immediately.
Duration of suspension
38 The suspension of a licence must be lifted if the Minister determines that corrective action has been taken.
Grounds for cancellation
39 The Minister may cancel a licence if
- (a) the licence holder fails to take corrective action within 90 days after the day on which the licence was suspended, unless a longer period is granted by the Minister at the written request of the licence holder;
- (b) the licence holder continues to conduct an activity that is identified in their licence while the licence is suspended;
- (c) the licence holder or any of their directors or officers has been convicted of an offence under the Act or the Food and Drugs Act;
- (d) the licence holder does not comply with any provision of the Act, other than section 15, or with any provision of these Regulations, the Food and Drugs Act or the Food and Drug Regulations and, since its issuance or renewal, the licence
- (i) has already been suspended for non-compliance with that provision, or
- (ii) has already been suspended twice; or
- (e) the licence holder was not in compliance with section 15 of the Act in respect of their application for the issuance, renewal or amendment of the licence or at any time during the period of validity of the licence.
Cancellation
40 (1) The Minister must not cancel a licence unless the licence holder was notified of the grounds for cancellation and was provided with an opportunity to be heard in respect of the cancellation.
Written notice
(2) The Minister must notify the licence holder in writing of the cancellation and the date on which it takes effect.
DIVISION 2
Inspection Services — Food Animals and Meat Products
Inspection stations — slaughtering
41 (1) The Minister must determine the number of inspection stations that are required annually during each work shift that has been approved by the President, for each establishment where food animals are slaughtered by a licence holder, taking into account the following factors:
- (a) the animal species that are slaughtered;
- (b) the method of carcass examination or inspection that is used;
- (c) the speed of the slaughter line; and
- (d) the volume of production.
Fixed or unfixed locations
(2) The Minister must determine whether inspection services at an inspection station are to be provided at a fixed or unfixed location in the establishment and, in the case of a fixed location, must specify the location in the establishment.
Additional inspection stations
(3) The Minister may authorize one or more additional inspection stations for a work shift, on an annual or hourly basis, taking into account the factors set out in subsection (1), if the licence holder submits a written request to the President and an inspector is available.
Minimum hours of inspection — meat products
42 The Minister must determine the minimum number of hours of inspection that are required per year during each work shift that has been approved by the President, for each establishment where a meat product is manufactured, processed, treated, preserved, graded, packaged or labelled, or where an edible meat product is stored and handled in its imported condition, by a licence holder, taking into account the following factors:
- (a) the nature and complexity of the activities that are conducted by the licence holder in the establishment;
- (b) the size of the establishment, the layout of equipment and the type of equipment and technology that are used;
- (c) the range of meat products and the volume of production;
- (d) work scheduling practices; and
- (e) the inspection records in respect of the establishment and the activities that are conducted by the licence holder in the establishment and, if available, any inspection records in respect of comparable establishments where the same activities are conducted.
Inspection services outside work shifts
43 Inspection services may be provided during a period other than a work shift if a licence holder submits a written request to the Minister and an inspector is available.
Notice
44 (1) A licence holder must notify the Minister in writing of any change that affects any of the factors set out in subsection 41(1) or section 42 or if an additional inspection station that is authorized under subsection 41(3) on an annual basis is no longer required.
Adjustment
(2) If the Minister becomes aware of a change referred to in subsection (1), the Minister must reconsider and, as appropriate, adjust the number of inspection stations or minimum number of hours of inspection that are required.
PART 4
Preventive Controls
DIVISION 1
Interpretation and Application
Definitions
45 The following definitions apply in this Part.
acceptable level means a level of a biological, chemical or physical hazard that does not present a risk of contamination of the food. (niveau acceptable)
agronomic input means an input that is used in growing fresh fruits or vegetables, and includes agricultural chemicals, biological controls, pollinators, commercial fertilizers, compost, compost tea, green manure, manure, mulch, row covers, soil amendments and pulp sludge. (intrant agronomique)
control measure means a measure that can be applied to prevent or eliminate any biological, chemical or physical hazard that presents a risk of contamination of a food or to reduce the hazard to an acceptable level. (mesure de contrôle)
critical control point means a step at which the application of a control measure is essential to prevent or eliminate any biological, chemical or physical hazard that presents a risk of contamination of a food or to reduce the hazard to an acceptable level. (point de contrôle critique)
low-acid food means a food of which any component has a pH that is greater than 4.6 and a water activity, as determined by the ratio of the water vapour pressure of the component to the vapour pressure of pure water at the same temperature and pressure, that is greater than 0.85. (aliment peu acide)
operator means
- (a) the holder of a licence to manufacture, process, treat, preserve, grade, store, package or label a food, to store and handle an edible meat product in its imported condition or to slaughter a food animal;
- (b) any person who grows or harvests fresh fruits or vegetables; and
- (c) any person who handles fish in a conveyance. (exploitant)
scheduled process means a process in which a treatment is applied to a food to render the food commercially sterile, taking into account the critical physical and chemical factors that affect the treatment’s effectiveness. (traitement programmé)
Application
46 (1) Unless otherwise specified, the requirements of this Part apply in respect of
- (a) any foods that are to be exported or sent or conveyed from one province to another;
- (b) any edible meat products that are imported, during their storing and handling in their imported condition, for the purpose of the exercise of an inspector’s powers under the Act; and
- (c) any food animals from which meat products that are to be exported or sent or conveyed from one province to another may be derived.
Exception
(2) Despite subsection (1), in the case of the holder of a licence to import, section 86 applies in respect of any food that is imported.
Application — establishment
(3) The requirements of this Part that refer to an establishment apply in respect of
- (a) in the case of the holder of a licence referred to in paragraph (a) of the definition operator in section 45, an establishment that is identified in their licence;
- (b) in the case of any person referred to in paragraph (b) of the definition operator in section 45, an establishment where that person grows or harvests fresh fruits or vegetables; and
- (c) in the case of any person referred to in paragraph (c) of the definition operator in section 45, the conveyance where that person handles fish.
Establishment — slaughtering game animals
(4) For the purposes of section 50, subsection 56(2) and sections 66, 67 and 71, in the case of an establishment that is identified in a licence to slaughter a game animal, the facility in the establishment is deemed to be the establishment.
Exception — game animals
(5) Section 55, subsection 56(1) and sections 58 and 69 do not apply in respect of an establishment where game animals are slaughtered.
DIVISION 2
Biological, Chemical and Physical Hazards
Identification and analysis of hazards
47 (1) An operator must identify and analyze the biological, chemical and physical hazards that present a risk of contamination of a food.
Prevention, elimination and reduction of hazards
(2) The operator must prevent, eliminate or reduce to an acceptable level the hazards referred to in subsection (1) by using control measures that are shown by evidence to be effective, including any treatment or process and including, in the case of a meat product, the control measures that are set out in the document entitled Preventive Control Requirements for Biological Hazards in Meat Products, prepared by the Agency and published on its website, as amended from time to time.
Imported food
(3) The holder of a licence to import must comply with subsections (1) and (2) in respect of a food that is imported.
DIVISION 3
Treatments and Processes
Application of scheduled process to low-acid food
48 (1) If a low-acid food is in a hermetically sealed package, an operator must apply the scheduled process referred to in subparagraph (3)(a)(i) and, if batch thermal treatment is applied, must use a temperature-sensitive indicator that visually indicates that the package has been thermally treated.
Exception — refrigerated or frozen food
(2) Subsection (1) does not apply if the low-acid food is kept refrigerated or frozen and the expressions “Keep Refrigerated” and “garder réfrigéré”, or “Keep Frozen” and “garder congelé”, as the case may be, are shown on the principal display panel.
Documents
(3) The operator must prepare documents that set out
- (a) in respect of each low-acid food,
- (i) a description of the scheduled process that will be applied to it, together with the name of the person who is responsible for developing the process, and
- (ii) the formulation of the food; and
- (b) in respect of each application of the scheduled process to a low-acid food,
- (i) the name of the food and its production volume,
- (ii) the equipment that is used for the treatment,
- (iii) any parameters of the treatment, such as start and end times, temperatures of the treatment and the pressure used in the treatment,
- (iv) a description of any maintenance of, and of any modifications to, the equipment that is used for the treatment,
- (v) any deviations from the scheduled process and any corrective action taken,
- (vi) the incubation results, and
- (vii) a description of any treatment of the cooling water.
Retention period of documents
(4) The documents that set out the information referred to in paragraph (3)(a) must be kept for three years after the day of the most recent application of the scheduled process to the low-acid food, and the documents that set out the information referred to in paragraph (3)(b) must be kept for three years after the day of the application of the scheduled process.
DIVISION 4
Maintenance and Operation of Establishment
SUBDIVISION A
Responsibility of Operator
Maintenance and operation
49 An operator must maintain and operate an establishment so that the requirements of sections 50 to 81 are met.
SUBDIVISION B
Sanitation, Pest Control and Non-food Agents
Clean and sanitary condition
50 (1) An establishment, and any conveyance or equipment in it that is used in connection with an activity that is regulated under the Act, must be clean and in a sanitary condition.
Cleaning and sanitation
(2) The cleaning and sanitation of the establishment and of any conveyance or equipment in it that is used in connection with an activity that is regulated under the Act must be conducted in a manner that does not present a risk of contamination of a food.
Animals — establishment
51 (1) An establishment must be protected against the entry of any animal that presents a risk of contamination of a food, except if, in the case of any land that forms part of an establishment, there are no reasonably practicable measures that may be taken to prevent the entry of such animals onto the land.
Animals — facility or conveyance
(2) An animal must not be in a facility or conveyance where a food is manufactured, prepared, stored, packaged or labelled or where a food animal is slaughtered, unless the animal is
- (a) a food that is intended to be manufactured, prepared, stored, packaged or labelled in the facility or conveyance;
- (b) a food animal that is intended to be slaughtered in the facility or conveyance, whether or not the meat products that may be derived from it are intended to be exported or to be sent or conveyed from one province to another; or
- (c) an animal that is intended to be used in the manufacturing or preparing of a food in the facility or conveyance.
Risk of contamination
(3) Any measures that are taken for the purposes of complying with subsections (1) and (2) must not present a risk of contamination of a food.
Sanitizers, agronomic inputs and non-food chemical agents
52 Any sanitizer, agronomic input or non-food chemical agent that is in an establishment must
- (a) be properly and clearly identified;
- (b) be suitable for its intended use and not present a risk of contamination of a food; and
- (c) be handled and used in a manner that does not present a risk of contamination of a food and that is in accordance with any manufacturer’s instructions.
SUBDIVISION C
Conveyances and Equipment
Conveyances and equipment — food
53 Any conveyance or equipment that is used in the manufacturing, preparing, storing, packaging or labelling of a food or in the slaughtering of a food animal must
- (a) be appropriate for the food or the food animal, as the case may be, and for the activity being conducted;
- (b) be designed, constructed and maintained to prevent contamination of the food;
- (c) be constructed of, and maintained using, materials that are suitable for their intended use and, if those materials present a risk of contamination of the food, that are
- (i) corrosion-resistant,
- (ii) durable,
- (iii) capable of withstanding repeated cleaning and, if necessary to prevent contamination of the food, repeated sanitizing, unless the equipment is intended for a single use, and
- (iv) free of any noxious constituent;
- (d) be equipped with instruments to control, indicate and record any parameters that are necessary to prevent contamination of the food;
- (e) function as intended;
- (f) be accessible and, if necessary for its cleaning, sanitizing, maintenance or inspection, able to be easily disassembled;
- (g) be used, maintained and, if necessary, calibrated in accordance with the manufacturer’s instructions and in a manner that does not present a risk of contamination of the food; and
- (h) have surfaces that, if they come into contact with a food, are smooth, free from pitting, cracks and flakes and non-absorbent, except when the surface does not present a risk of contamination of the food.
Other conveyances and equipment
54 Any conveyance or equipment in an establishment that is used to handle any contaminated materials, any waste or any other thing that is inedible must, unless that conveyance or equipment does not come into contact with those materials, waste or things,
- (a) be used only for that purpose;
- (b) be identified as being reserved for that purpose; and
- (c) meet the applicable requirements of section 53.
Equipment — restraining
55 An establishment where food animals are slaughtered must have equipment for restraining food animals during their handling, their assessment, their ante-mortem examination and their inspection.
SUBDIVISION D
Conditions Respecting Establishments
Land
56 (1) If any land that forms part of an establishment presents a risk of contamination of a food, measures must be taken to eliminate the risk.
Location
(2) If an establishment is located near any place or thing that presents a risk of contamination of a food, measures must be taken to eliminate the risk.
Interior of facility or conveyance
57 The interior of any facility or conveyance where a food is manufactured, prepared, stored, packaged or labelled or where a food animal is slaughtered must be
- (a) designed to prevent the accumulation of substances that present a risk of contamination of the food, including dust, dirt, micro-organisms and food particles, and to permit effective maintenance, cleaning and sanitizing;
- (b) designed, constructed and maintained in such a manner that
- (i) the size and layout is adequate to accommodate the activity being conducted and the equipment used in the activity,
- (ii) the entry of insects, rodents and other vermin is prevented,
- (iii) any floors, walls, ceilings, windows and doors are smooth, non-absorbent and impervious to moisture, except if those floors, walls, ceilings, windows or doors do not present a risk of the contamination of the food, and
- (iv) any floors provide or permit good drainage, except if there is no risk of liquid accumulation;
- (c) constructed of, and maintained using, materials that are
- (i) suitable for their intended use,
- (ii) appropriate for the food or the food animal, as the case may be, and for the activity being conducted,
- (iii) durable,
- (iv) capable of withstanding repeated cleaning and, if necessary to prevent contamination of the food, repeated sanitizing, and
- (v) free of any noxious constituent; and
- (d) of sound construction and in good repair.
Slaughtering — separate areas
58 (1) An establishment where food animals are slaughtered must have separate areas for
- (a) keeping, examining and inspecting food animals;
- (b) segregating and isolating food animals under section 132 or paragraph 140(b);
- (c) holding food animals that show a deviation from normal behaviour, physiology or appearance; and
- (d) humanely killing food animals under paragraph 140(c).
Inedible meat products area
(2) The establishment must also have an enclosed area for the handling of inedible meat products.
Movement of food animals
(3) Floors, ramps, gangways and chutes that are used by food animals in the establishment must provide secure footing and must not present a risk of injury to the food animals during movement.
Stations for inspections, examinations and screenings
(4) The establishment must be equipped with inspection stations at the fixed locations specified by the President under subsection 41(2) and in the numbers determined by the President under subsection 41(1), and
- (a) if a licence holder is authorized to conduct a post-mortem examination program, stations for post-mortem examinations; or
- (b) if a licence holder is authorized to conduct a post-mortem defect management program, stations for post-mortem screenings.
Design, construction and maintenance — movement
59 (1) A facility or conveyance where a food is manufactured, prepared, stored, packaged or labelled or where a food animal is slaughtered must be designed, constructed and maintained in such a manner that the movement of persons and things within, into and out of it is controlled.
Movement — no risk of contamination
(2) The movement must not present a risk of contamination of the food.
Incompatible activities
60 Physical or other effective means must be used to separate incompatible activities in order to prevent contamination of a food.
Separation of food
61 Physical or other effective means must be used to separate a food from
- (a) anything that presents a risk of contamination of the food;
- (b) any food that does not meet the requirements of the Act or these Regulations; and
- (c) anything that is manufactured, prepared, stored, packaged or labelled in an establishment and not intended or sold for use as food.
Arrival of certain food at establishment
62 (1) Any food that presents a risk of injury to human health, that is exempted under section 22 from the application of the import requirements that are set out in the Act and these Regulations or that does not meet the requirements that are set out in the Act or these Regulations must be identified as such and placed in a designated area when it arrives at an establishment.
Measures to prevent contamination
(2) Any measures that are necessary to prevent the food described in subsection (1) from contaminating any other food that is in the establishment must be taken.
Lighting
63 (1) An establishment must be equipped with natural or artificial lighting that is appropriate for the food or the food animal that is intended to be slaughtered, as the case may be, and for the activity being conducted.
Light fixtures
(2) Any light fixtures in the establishment must
- (a) be capable of withstanding repeated cleaning and, if necessary to prevent contamination of a food, repeated sanitizing; and
- (b) not present a risk of contamination of the food in the event of breakage.
Ventilation system
64 A facility or conveyance where a food is manufactured, prepared, stored, packaged or labelled or where a food animal is slaughtered must be equipped with a ventilation system that
- (a) provides natural or mechanical ventilation with sufficient air exchange to provide clean air and to remove unclean air and odours that might affect the food;
- (b) is accessible and, if necessary for its cleaning, maintenance or inspection, is able to be disassembled;
- (c) is capable of withstanding repeated cleaning; and
- (d) functions as intended.
Temperature and humidity
65 (1) The temperature and humidity level in a facility or conveyance where a food is manufactured, prepared, stored, packaged or labelled or where a food animal is slaughtered must be maintained at levels that are appropriate for the food or the food animal, as the case may be, and for the activity being conducted.
Heating, cooling or humidity-control system
(2) If the facility or conveyance is equipped with a heating, cooling or humidity-control system, the system must
- (a) if necessary to prevent contamination of a food, be equipped with instruments to control, indicate and record the temperature and humidity levels;
- (b) be accessible and, if necessary for its cleaning, maintenance or inspection, is able to be disassembled;
- (c) be capable of withstanding repeated cleaning; and
- (d) function as intended.
Removal and disposal of contaminated materials and waste
66 (1) An establishment must have means for the removal and disposal of contaminated materials and waste and, if necessary to prevent contamination of a food, be equipped with a drainage, sewage and plumbing system that functions as intended.
Frequency and manner
(2) Contaminated materials and waste must be removed and disposed of at a frequency that is sufficient to prevent contamination of a food and in a manner that does not present a risk of contamination of a food.
Cleaning stations, lavatories, etc.
67 (1) If necessary to prevent the contamination of a food, an establishment must be equipped with hand cleaning and sanitizing stations, lavatories, showers, drinking water stations, break rooms or change rooms that
- (a) are appropriately equipped and adequate in number and size for the number of persons using them;
- (b) are located so that they are readily accessible to the persons using them; and
- (c) are capable of withstanding repeated cleaning and, if necessary to prevent contamination of a food, repeated sanitizing.
Hand cleaning and sanitizing stations
(2) The hand cleaning and sanitizing stations must permit the effective cleaning of hands.
Lavatories
(3) The lavatories must be located and maintained so that they do not present any risk of contamination of a food.
Area for inspector’s use
68 At the request of an inspector, the inspector must be provided with an area that is readily accessible, appropriately equipped and appropriate in size for the exercise of their powers, and the performance of their duties and functions, under the Act.
Office, lockers, etc., for inspector
69 (1) An establishment in which a meat product or processed egg product is manufactured, prepared, stored, packaged or labelled or in which a food animal is slaughtered must provide an inspector with
- (a) a furnished office that is readily accessible, appropriately equipped and appropriate in size for the exercise of their powers and the performance of their duties and functions under the Act;
- (b) lockers and cabinets that are readily accessible and appropriate for the protection and storing of their equipment and documents; and
- (c) access to a lockable storage facility or equipment that is appropriate for the protection, preservation and storing of samples.
Private office, change rooms, etc., for inspector
(2) In the case of an establishment in which a food animal is slaughtered, the office referred to in paragraph (1)(a) must be private and the inspector must also be provided with access to a lavatory, a shower and a change room in the establishment.
Water — contact with food
70 (1) Any water that might come into contact with a food must be potable, unless it does not present a risk of contamination of the food, and must be protected against contamination.
Steam and ice — contact with food
(2) Any steam or ice that might come into contact with a food must be made from water that meets the requirements of subsection (1), unless the steam or ice does not present a risk of contamination of the food.
Water — cross-connections
(3) Any system that supplies water that meets the requirements of subsection (1) must not be cross-connected with any other system, unless measures are taken to eliminate any risk of contamination of a food as a result of the cross-connection.
Water given to food animals
(4) Any water or other source of hydration that is provided to food animals that are intended to be slaughtered in an establishment must not present a risk of injury to the health of those animals and must not present a risk of contamination of the meat products that may be derived from those animals.
Supply of water, steam and ice
71 (1) An establishment must be supplied, as appropriate for the food or the food animal that is intended to be slaughtered, as the case may be, and for the activity being conducted, with
- (a) water that is adequate in quantity, temperature, pH and pressure to meet the needs of the establishment;
- (b) steam that is adequate in quantity and pressure to meet those needs; and
- (c) ice that is adequate in quantity to meet those needs.
Treatment of water, steam or ice
(2) Any treatment of water, steam or ice must be applied in a manner that does not present a risk of contamination of a food.
SUBDIVISION E
Unloading, Loading and Storing
Conveyances
72 Any conveyance that is used to convey a food to or from an establishment and that is unloaded or loaded at the establishment
- (a) must be designed, constructed and maintained to prevent contamination of the food;
- (b) must be constructed of, and maintained using, materials that are suitable for their intended use and, if the materials present a risk of contamination of the food, that are
- (i) durable,
- (ii) capable of withstanding repeated cleaning and, if necessary to prevent contamination of a food, repeated sanitizing, and
- (iii) free of any noxious constituent;
- (c) must be capable of maintaining the temperature and humidity at levels that are appropriate for the food and, if necessary to prevent contamination of the food, be equipped with instruments that control, indicate and record those levels;
- (d) must not contain any animal, other than an animal referred to in paragraph 51(2)(a), any pest control product as defined in subsection 2(1) of the Pest Control Products Act or any other material or substance that presents a risk of contamination of the food; and
- (e) must be clean and in a sanitary condition at the time of unloading or loading.
Unloading and loading
73 Any unloading and loading of a food or of a food animal that is intended to be slaughtered, from or onto a conveyance at an establishment, must be conducted in a manner that does not present a risk of contamination of a food.
Storing — food
74 (1) Any storing of a food must be conducted in a manner that does not present a risk of contamination of the food.
Storing — other
(2) Any storing of conveyances, equipment, sanitizers, agronomic inputs, chemical agents, starter products, packaging material, labels or any other thing that is used in the manufacturing, preparing, storing, packaging or labelling of a food must be conducted in a manner that does not present a risk of contamination of the food.
Definition of starter products
(3) In subsection (2), starter products means the materials that are used to start growing fresh fruits or vegetables and includes seeds, seedlings, plants, cuttings, canes, seed potatoes and nursery stock.
SUBDIVISION F
Competency
Competencies and qualifications
75 Any person who is involved in the manufacturing, preparing, storing, packaging or labelling of a food or in the slaughtering of a food animal must have the competencies and qualifications that are necessary to carry out their duties.
SUBDIVISION G
Hygiene
Clothing, footwear and protective coverings
76 Any person who enters or is in an area where a food is manufactured, prepared, stored, packaged or labelled or where a food animal is slaughtered must wear clothing, footwear and protective coverings, including gloves, a hairnet, a beard net and a smock, that are in good condition, clean and in sanitary condition and that are appropriate for the food and for the activity being conducted.
Personal cleanliness
77 Any person who enters or is in an area where a food is manufactured, prepared, stored, packaged or labelled or where a food animal is slaughtered must maintain personal cleanliness to prevent contamination of the food, including by cleaning and, if necessary, by sanitizing their hands
- (a) immediately on entering the area;
- (b) immediately after using a lavatory;
- (c) immediately before beginning to conduct the activity; and
- (d) at a frequency appropriate for the food and for the activity being conducted.
Spitting, chewing gum and other acts
78 Any person who enters or is in an area where a food is manufactured, prepared, stored, packaged or labelled or where a food animal is slaughtered must refrain from spitting, chewing gum, using tobacco products, eating, having unnecessary contact with the food and doing any other act that presents a risk of contamination of the food.
Objects and substances — risk of contamination
79 Any person who enters or is in an area where a food is manufactured, prepared, stored, packaged or labelled or where a food animal is slaughtered must refrain from wearing or using any object or substance that presents a risk of contamination of the food.
Reporting of disease, illness, symptoms and lesions
80 Any person who works in an area where a food is manufactured, prepared, stored, packaged or labelled or where a food animal is slaughtered and who has a disease or illness, symptoms of a disease or illness or an open or infected lesion must report them to the operator.
Communicable disease and lesions — risk of contamination
81 The operator must prevent any person who is suffering from, or is a known carrier of, a communicable disease or who has an open or infected lesion from entering or being in an area of an establishment where a food is manufactured, prepared, stored, packaged or labelled or where a food animal is slaughtered if the person’s condition presents a risk of contamination of the food.
DIVISION 5
Investigation, Notification, Complaints and Recall
Investigation
82 (1) An operator who suspects on reasonable grounds that a food presents a risk of injury to human health or does not meet the requirements of the Act or these Regulations must immediately investigate the matter.
Notification and mitigation of risk
(2) If the investigation establishes that the food presents a risk of injury to human health, the operator must immediately notify the Minister and immediately take action to mitigate the risk.
Complaints procedure
83 (1) An operator must prepare, keep and maintain a document that sets out a procedure for receiving, investigating and responding to complaints that are received in relation to a food.
Complaints
(2) If a complaint is received, the operator must implement the procedure and prepare a document that sets out the details of the complaint, the results of the investigation and the actions taken based on those results and keep it for two years after the day on which the actions are completed.
Recall procedure
84 (1) An operator must prepare, keep and maintain a document that sets out a recall procedure that enables the effective recall of a food, the name of a contact person who is responsible for the procedure and the name of a contact person who is responsible for conducting recalls.
Recall simulation
(2) The operator must, at least once every 12 months,
- (a) conduct a recall simulation, based on the recall procedure; and
- (b) prepare a document that sets out the details of how the recall simulation was conducted and the results of the simulation, and keep that document for two years after the day on which the recall simulation is completed.
Recall — notice to Minister
(3) If an operator determines that a food should be recalled because it presents a risk of injury to human health, the operator must immediately notify the Minister.
Recall — implementation
(4) If a food is the subject of a recall because it presents a risk of injury to human health, the operator must
- (a) immediately implement the recall procedure; and
- (b) prepare a document that sets out the details of the recall, including any information that substantiates its effectiveness, and keep the document for two years after the day on which the recall is initiated.
Imported food
85 The holder of a licence to import must comply with sections 82 to 84 in respect of a food that is imported.
DIVISION 6
Preventive Control Plan
Licence holders
86 (1) A licence holder must prepare, keep and maintain a written preventive control plan that meets the requirements of section 89 for any activity identified in their licence that they conduct in respect of a food or food animal.
Exception — food to be exported
(2) Despite subsection (1), a preventive control plan is not required to be prepared, kept or maintained for any activity that the licence holder conducts in respect of a food, other than fish or a meat product, that is exported, unless a certificate or other document referred to in section 48 of the Act is sought in respect of the food.
Exception — sales of $100,000 or less
(3) Despite subsection (1), if a licence holder’s gross sales that are derived from food are $100,000 or less for the 12 months before the day on which they most recently made an application for the issuance, renewal or amendment of a licence, a preventive control plan must be prepared, kept and maintained only for any activity that they conduct in respect of
- (a) a food animal, meat product, fish, dairy product, egg, processed egg product or processed fruit or vegetable product that is identified in their licence; and
- (b) a food in respect of which a certificate or other document referred to in section 48 of the Act is sought.
Growers or harvesters of fresh fruits or vegetables
87 Any person who grows or harvests fresh fruits or vegetables must prepare, keep and maintain a written preventive control plan that meets the requirements of section 89 for any activity that they conduct in respect of those fresh fruits or vegetables if they are
- (a) to be exported and a certificate or other document referred to in section 48 of the Act is sought in respect of the fresh fruits or vegetables; or
- (b) to be sent or conveyed from one province to another and the person’s gross sales that are derived from food are more than $100,000 for the previous 12 months.
Implementation
88 Any person who is required to prepare, keep and maintain a preventive control plan must implement that plan.
Content of preventive control plan
89 (1) The preventive control plan must include
- (a) a description of the measures for ensuring that the applicable requirements of sections 201 and 205, subsection 206(1), sections 208, 218, 221, 296, 306, 307, 316, 317, 321, 322, 324 to 326 and 328 are met;
- (b) a description of the measures for ensuring that the food is packaged and labelled in a manner that does not contravene subsection 6(1) of the Act;
- (c) in relation to the applicable requirements of these Regulations,
- (i) a description of the biological, chemical and physical hazards that are identified under subsection 47(1) as presenting a risk of contamination of a food, of the control measures for preventing or eliminating those hazards or reducing them to an acceptable level and of the evidence that the control measures are effective,
- (ii) a description of the critical control points, of the related control measures and of the evidence that the control measures are effective,
- (iii) a description of the critical limits for each critical control point,
- (iv) the procedures for monitoring the critical control points in relation to their critical limits,
- (v) the corrective action procedures for each critical control point,
- (vi) the procedures for verifying that the implementation of the preventive control plan results in compliance with the provisions of the Act and these Regulations, and
- (vii) documents that substantiate that the preventive control plan has been implemented with respect to subparagraphs (i) to (vi); and
- (d) in relation to the applicable requirements of sections 128 to 136, paragraphs 140(b) and (c) and sections 141 to 144,
- (i) a description of the measures for preventing or eliminating a risk of avoidable suffering, injury or death to the food animals during their handling, and of the evidence that those measures are effective,
- (ii) a description of the measures for preventing or eliminating a risk of avoidable suffering or injury to the food animals during their slaughtering, and of the evidence that those measures are effective,
- (iii) a description of the performance criteria for evaluating the effectiveness of each of those measures,
- (iv) the procedures for monitoring each of those measures,
- (v) the corrective action procedures for each of those measures,
- (vi) the procedures for verifying that the implementation of the preventive control plan results in compliance with the provisions of the Act and these Regulations,
- (vii) the procedures for auditing, on a regular basis, the outcome of the implementation of the preventive control plan, and
- (viii) documents that substantiate that the preventive control plan has been implemented with respect to subparagraphs (i) to (vii); and
- (e) supporting documents that show evidence of the information recorded under paragraphs (a) and (b), subparagraphs (c)(i) to (vi) and (d)(i) to (vii).
Retention period of documents
(2) Each document referred to in subparagraphs (1)(c)(vii) and (d)(viii) must be kept for two years after the day on which it is prepared.
Exception — game animals
(3) The preventive control plan of the holder of a licence to slaughter a game animal is not required to include any information specified in subsection (1) other than the information specified in subparagraphs (1)(c)(i) and (d)(i).
Additional content — import
(4) The preventive control plan of the holder of a licence to import must also include the information specified in subparagraphs (1)(c)(i) to (vii) in relation to the requirements of section 11.
Additional content — export
(5) The preventive control plan of the holder of a licence to export must also include the information specified in subparagraphs (1)(c)(i) to (vii) in relation to the requirements of subsection 15(1).
Additional content — post-mortem programs
(6) The preventive control plan of a licence holder who is authorized under subsection 160(3) to conduct a post-mortem examination program or a post-mortem defect management program must also include the information specified in subparagraphs (1)(c)(i) to (vii) and paragraph (1)(e) in relation to that program and must meet the requirements that are set out in
- (a) in the case of a port-mortem examination, the document entitled Fundamentals of the Post-mortem Examination Program, prepared by the Agency and published on its website, as amended from time to time; or
- (b) in the case of a post-mortem defect management program, the document entitled Fundamentals of the Post-mortem Defect Management Program, prepared by the Agency and published on its website, as amended from time to time.
PART 5
Traceability
Documents
90 (1) Any person who sends or conveys a food from one province to another, or who imports or exports it, any holder of a licence to slaughter a food animal, to manufacture, process, treat, preserve, grade, store, package or label a food or to store and handle an edible meat product in its imported condition and any person who grows or harvests fresh fruits or vegetables that are to be sent or conveyed from one province to another or exported must, if they provide the food to another person, prepare and keep documents that set out
- (a) the common name of the food, a lot code or other unique identifier that enables the food to be traced and the name and principal place of business of the person by or for whom the food was manufactured, prepared, produced, stored, packaged or labelled;
- (b) except if they provide the food to another person as a sale at retail, the date on which it was provided and the name and address of the person to whom it was provided;
- (c) if they were provided the food by another person, the name and address of that person and the date on which it was provided; and
- (d) the name of any food commodity that they incorporated into the food or from which they derived the food and, if they were provided the food commodity by another person, the name and address of that person and the date on which it was provided.
Documents — retail sale
(2) Any person who sells a food at retail, other than a restaurant or other similar enterprise that sells the food as a meal or snack, must prepare and keep documents that include the information specified in paragraphs (1)(a), (c) and (d).
Retention period of documents
(3) The documents referred to in subsections (1) and (2) must be kept for two years after the day on which the food was provided to another person or sold at retail, and must be accessible in Canada.
Production of documents
91 (1) Any person who has received a request from the Minister for a document referred to in section 90, or any part of such a document, must provide it to the Minister
- (a) within 24 hours after receipt of the request, or within
- (i) any shorter period that is specified by the Minister, if the Minister believes that it is necessary in order to identify or respond to a risk of injury to human health associated with a food commodity, or
- (ii) any longer period that is specified by the Minister, if the Minister believes that the document is not necessary for a recall that is or may be ordered under subsection 19(1) of the Canadian Food Inspection Agency Act; and
- (b) if provided electronically, in a single file and in plain text that is capable of being imported into and manipulated by standard commercial software.
Definition of plain text
(2) In paragraph (1)(b), plain text means data that is not encrypted and whose semantic content is available.
Labelling
92 (1) Any person referred to in subsection 90(1) or (2) must ensure that a label that bears the information specified in paragraph 90(1)(a) is applied or attached to any food, or accompanies any food, that is provided to another person.
Consumer prepackaged food
(2) In the case of consumer prepackaged food that is not packaged at retail, the unique identifier referred to in paragraph 90(1)(a) must be a lot code.
Exception
(3) Subsections (1) and (2) do not apply in respect of
- (a) a food at the time of its export;
- (b) a food, other than a consumer prepackaged food, at the time of its sale at retail; or
- (c) a prepackaged food described in paragraphs 213(a) to (c) at the time of its sale at retail.
Exception — foods described in paragraphs 219(1)(a) and (b)
(4) Despite subsection (1), any food described in paragraph 219(1)(a) or (b) is not required to be labelled with the common name of the food at the time of its sale at retail.
Exception — foods described in section 220
(5) Despite subsection (1), any food described in section 220 is not required to be labelled with the name and principal place of business of the person by or for whom the food was manufactured, prepared, produced, stored, packaged or labelled.
PART 6
Commodity-specific Requirements
DIVISION 1
Application
Application — import, interprovincial trade and export
93 (1) The requirements of this Part apply in respect of
- (a) any foods that have been imported or any foods that are to be sent or conveyed from one province to another or exported; and
- (b) any food animals from which meat products that are to be sent or conveyed from one province to another or exported may be derived.
Application — food animals
(2) The requirements of sections 128 to 136 apply in respect of food animals that are in an establishment that is identified in a licence to slaughter.
DIVISION 2
Dairy Products
Preparation
94 Any milk or cream that is used in preparing a dairy product that is to be sent or conveyed from one province to another or exported must meet the applicable requirements of the legislation of the province in which the dairy product is prepared.
DIVISION 3
Eggs
Pasteurization
95 (1) A licence holder may pasteurize eggs in the shell only if they are graded Canada A or Grade A.
Import — eggs pasteurized in shell
(2) Eggs that are pasteurized in the shell and that are imported must have been graded Grade A before pasteurization.
Import — foreign official document
96 The holder of a licence to import may import eggs only if the licence holder provides an inspector with an official document issued by the foreign state, in a form approved by the President, that states that the eggs meet the requirements that are set out in the Act and these Regulations.
Import — Grade C or Grade Nest Run
97 A licence holder who imports eggs that are graded Grade C or Grade Nest Run must deliver them directly to an establishment where eggs are processed and treated by a licence holder.
Import — ungraded eggs
98 (1) Despite subsection 306(1), a licence holder may import ungraded eggs if they
- (a) before the import, notify the Minister in writing of the quantity of ungraded eggs that are intended to be imported, the date of the import and the name of the licence holder and address of the establishment referred to in paragraph (c);
- (b) package them in a container that is labelled with the expression “Ungraded Eggs” or “œufs non classifiés”; and
- (c) deliver them directly to an establishment where eggs are processed and treated by a licence holder.
Removal — imported ungraded eggs
(2) Any imported ungraded eggs delivered to an establishment referred to in paragraph (1)(c) may be removed from that establishment if
- (a) the eggs have been processed and treated by a licence holder; or
- (b) the eggs are delivered directly to another establishment where eggs are processed and treated by a licence holder.
Interprovincial trade
99 (1) Any person who sends or conveys any of the following from one province to another must deliver them to an establishment where eggs are processed and treated by a licence holder:
- (a) eggs that are graded Canada A or Canada B that bear an ink mark that consists of the word “dyed” or “teint” or a deposit of ink that is applied to an egg’s shell by the holder of a licence to grade eggs;
- (b) eggs that are graded Canada C;
- (c) eggs that are graded Grade C or Grade Nest Run that are imported; and
- (d) ungraded eggs that are imported in accordance with subsection 98(1).
Interprovincial trade — Canada Nest Run
(2) Any person who sends or conveys eggs that are graded Canada Nest Run from one province to another must deliver them to an establishment where either eggs are graded or eggs are processed and treated by a licence holder.
Interprovincial trade — ungraded eggs
(3) Despite subsection 306(1), any person may send or convey from one province to another ungraded eggs, other than eggs that are rejected in accordance with subsection 333(1) or eggs that are imported in accordance with subsection 98(1), if the person
- (a) packages them in a container that is labelled with the expression “Ungraded Eggs” or “œufs non classifiés”; and
- (b) delivers them to an establishment where either eggs are graded or eggs are processed and treated by a licence holder.
Ink
100 If a licence holder applies ink to an egg’s shell, the ink must be fast-drying and indelible and it must not present a risk of injury to human health.
Trays
101 Before sending plastic trays to an egg producer, a licence holder must clean, sanitize and dry them.
DIVISION 4
Processed Egg Products
Processing and treating eggs
102 (1) A licence holder may process and treat eggs only if they
- (a) are edible;
- (b) do not emit an abnormal odour;
- (c) are not mouldy;
- (d) have not been in an incubator;
- (e) do not have an internal defect, other than a particle of the oviduct or a blood spot neither of which exceeds 3 mm in diameter;
- (f) are not leakers, except if they become leakers while being transferred to the egg-breaking equipment and they are prepared in a manner that prevents the contamination of the processed egg product; and
- (g) are free from dirt and other foreign matter.
Processing and treating processed egg products
(2) A licence holder may process and treat processed egg products only if they are derived from eggs that meet the requirements of paragraphs (1)(a) to (g).
Temperature
103 (1) A processed egg product that is to be sent or conveyed from one province to another or exported and that is processed or treated in an establishment that is identified in a licence must have been chilled to 4°C or less before being removed from the establishment, if it is a
- (a) liquid whole egg;
- (b) liquid yolk;
- (c) liquid egg white or liquid albumen;
- (d) liquid whole egg mix;
- (e) liquid yolk mix; and
- (f) liquid egg product.
Exception
(2) Despite subsection (1), the Minister may, in writing, authorize any person to remove a processed egg product that has not been chilled to 4°C or less if the Minister is of the opinion that no risk of injury to human health will result.
Import — foreign official document
104 The holder of a licence to import may import a processed egg product only if the licence holder provides an inspector with an official document issued by the foreign state, in a form approved by the President, that states that the processed egg product meets the requirements that are set out in the Act and these Regulations.
DIVISION 5
Fish
Prohibition — import of mitten crab or puffer fish
105 (1) It is prohibited for a person to import
- (a) a live, freshwater mitten crab of the genus Eriocheir; or
- (b) a puffer fish of the family Tetraodontidae.
Exception — personal use
(2) Section 19 of the Act does not apply in respect of an import referred to in subsection (1).
Import of live or raw shellfish
106 (1) The holder of a licence to import may import live or raw shellfish only if
- (a) the foreign state in which the shellfish is harvested, manufactured, prepared, stored, packaged or labelled, as the case may be, has, at the time the activity is conducted, an inspection system for the shellfish that is recognized under Part 7;
- (b) the foreign state from which the shellfish is imported has, at the time of the import, an inspection system for shellfish that is recognized under Part 7; and
- (c) any establishment where the shellfish was manufactured, prepared, stored, packaged or labelled has, at the time that the activity is conducted and at the time of the import, a system for manufacturing, preparing, storing, packaging or labelling, as the case may be, that complies with an inspection system for shellfish that is recognized under Part 7.
Exception
(2) The conditions in paragraphs (1)(a) to (c) do not apply in respect of the import of the adductor muscles of scallops or the meat of geoducks.
Shellfish
107 (1) A licence holder may manufacture, prepare, store, package or label shellfish that is to be exported or sent or conveyed from one province to another only if the shellfish is harvested in an area
- (a) that has been classified under the Canadian Shellfish Sanitation Program and that is not subject to an order prohibiting fishing issued under the Management of Contaminated Fisheries Regulations; or
- (b) that is subject to an order prohibiting fishing issued under the Management of Contaminated Fisheries Regulations but the shellfish has been decontaminated in accordance with the decontamination plan submitted in connection with a licence to fish for food purposes issued under those Regulations.
Exception
(2) The conditions in paragraphs (1)(a) and (b) do not apply in respect of the manufacturing, preparing, storing, packaging or labelling of the adductor muscles of scallops or the meat of geoducks.
Frozen fish
108 A licence holder must protect from dehydration and oxidation all frozen fish that is stored in a conveyance.
DIVISION 6
Fresh Fruits or Vegetables
SUBDIVISION A
Interpretation and Application
Definitions
109 The following definitions apply in this Division.
apple means a fresh apple for which a grade is prescribed by these Regulations. (pomme)
onion means a fresh onion for which a grade is prescribed by these Regulations. (oignon)
potato means a fresh potato for which a grade is prescribed by these Regulations. (pomme de terre)
Fresh fruits or vegetables packaged together
110 The requirements of sections 113 to 121 and 269 — as well as any requirements under Division 2 of Part 10 and Part 12 that apply in respect of fresh fruits or vegetables — do not apply in respect of consumer prepackaged fresh fruits or vegetables if the container contains more than one type of fresh fruit or vegetable but no other food and if
- (a) the label that is applied or attached to the container bears the expression “Fresh Pack” or “emballage frais” or, in the case of consumer prepackaged fresh vegetables, the expression “Stew-pack” or “légumes mixtes” or the expression “Vegetables for Stew” or “légumes pour ragoût”;
- (b) no one type of fresh fruit or vegetable in the container exceeds 1 kg net weight; and
- (c) the net weight of the fresh fruits or vegetables in the container does not exceed 10 kg.
Fresh fruits or vegetables packaged with other food
111 The requirements of sections 113 to 121 and 269 — as well as any requirements under Division 2 of Part 10 and Part 12 that apply in respect of fresh fruits or vegetables — do not apply in respect of consumer prepackaged fresh fruits or vegetables if the container contains more than one type of fresh fruit or vegetable together with other food and if
- (a) the label that is applied or attached to the container bears the expression “Gift Pack” or “emballage-cadeau” or the expression “Combo Pack” or “emballage mixte”;
- (b) no one type of fresh fruit or vegetable in the container exceeds 1 kg net weight; and
- (c) the net weight of the fresh fruits or vegetables and other food in the container does not exceed 10 kg.
SUBDIVISION B
Import
Whole fresh fruits or vegetables
112 The requirements of this Subdivision apply in respect of any fresh fruits or vegetables that are whole.
Imported potatoes
113 (1) Potatoes that are imported must meet the requirements for the grade Canada No. 1 that are set out in the Compendium.
Presumption — potatoes from United States
(2) Potatoes that are imported from the United States are considered to meet the requirements for the grade Canada No. 1 that are set out in the Compendium if the potatoes have been graded in the United States and meet the applicable requirements that are set out in the document entitled Grade Standard Requirements for Fresh Fruits or Vegetables Imported from the United States, prepared by the Agency and published on its website, as amended from time to time.
Apples from foreign state other than United States
114 (1) Apples that are imported from a foreign state other than the United States must meet the requirements for the grade Canada Extra Fancy, Canada Fancy or Canada Commercial that are set out in the Compendium.
Apples from United States
(2) Apples that are imported from the United States must meet the requirements for the grade Canada Extra Fancy or Canada Fancy that are set out in the Compendium.
Presumption — apples from United States
(3) Apples that are imported from the United States are considered to meet the requirements for the grade Canada Extra Fancy or Canada Fancy that are set out in the Compendium if the apples have been graded in the United States and meet the applicable requirements that are set out in the document entitled Grade Standard Requirements for Fresh Fruits or Vegetables Imported from the United States, prepared by the Agency and published on its website, as amended from time to time.
Presumption — general
115 Fresh fruits or vegetables, other than potatoes or apples, that are imported from the United States are considered to meet the applicable requirements that are set out in the Compendium if the fruits or vegetables have been graded in the United States and meet the applicable requirements that are set out in the document entitled Grade Standard Requirements for Fresh Fruits or Vegetables Imported from the United States, prepared by the Agency and published on its website, as amended from time to time.
Foreign states — onions, potatoes and apples
116 Onions and potatoes that are imported from a foreign state other than the United States, and apples that are imported from a foreign state other than the United States and New Zealand, must meet and be certified by the Minister as meeting the following requirements:
- (a) the applicable requirements that are set out in Parts 10 to 12;
- (b) in the case of onions, the requirements for a particular grade of onions that are set out in the Compendium;
- (c) in the case of potatoes, the requirements for the grade Canada No. 1 that are set out in the Compendium; and
- (d) in the case of apples, the requirements for the grade Canada Extra Fancy, Canada Fancy or Canada Commercial that are set out in the Compendium.
Onions, potatoes and apples from United States
117 (1) Onions, potatoes and apples that are imported from the United States must
- (a) be accompanied at the Canadian port of entry by a serially numbered certificate or evidence, in the form of a facsimile or a copy of an email message, issued by the federal department responsible for agriculture in the United States, that establishes that the following requirements are met:
- (i) the applicable requirements that are set out in Parts 10 to 12,
- (ii) in the case of onions, the requirements for a particular grade of onions that are set out in the Compendium,
- (iii) in the case of potatoes, the requirements for the grade Canada No. 1 that are set out in the Compendium, and
- (iv) in the case of apples, the requirements for the grade Canada Extra Fancy or Canada Fancy that are set out in the Compendium; or
- (b) meet and be certified by the Minister as meeting the requirements that are set out in subparagraph (a)(i) and, in accordance with any general tolerances for inspection at the time of shipping or repackaging that are set out in the Compendium, the requirements that are set out in subparagraph (a)(ii), (iii) or (iv).
Endorsement
(2) The certificate and evidence referred to in paragraph (1)(a) must be endorsed with the expression “Meets Canadian Import Requirements for Grades, Packaging, Labelling and Standard Container Size” or “satisfait aux exigences d’importation du Canada visant la classification, l’emballage, l’étiquetage et la taille des contenants normalisés”.
Apples from New Zealand
118 (1) Apples that are imported from New Zealand must
- (a) be accompanied at the Canadian port of entry by a serially numbered certificate or evidence, in the form of a facsimile or a copy of an email message, issued by the ministry responsible for agriculture in New Zealand, that establishes that the following requirements are met:
- (i) the applicable requirements that are set out in Parts 10 to 12, and
- (ii) the requirements for the grade Canada Extra Fancy, Canada Fancy or Canada Commercial that are set out in the Compendium; or
- (b) meet and be certified by the Minister as meeting the requirements that are set out in subparagraph (a)(i) and, in accordance with any general tolerances for inspection at the time of shipping or repackaging that are set out in the Compendium, the requirements that are set out in subparagraph (a)(ii).
Endorsement
(2) The certificate and evidence referred to in paragraph (1)(a) must be endorsed with the expression “Meets Canadian Import Requirements for Grades, Packaging, Labelling and Standard Container Size” or “satisfait aux exigences d’importation du Canada visant la classification, l’emballage, l’étiquetage et la taille des contenants normalisés”.
Exception
119 Sections 116 to 118 do not apply in respect of onions, potatoes or apples that are part of a shipment that consists of not more than
- (a) 15 containers of onions with an aggregate net weight of not more than 250 kg of onions;
- (b) 15 containers of potatoes with an aggregate net weight of not more than 250 kg of potatoes; and
- (c) 15 containers of apples with an aggregate net weight of not more than 250 kg of apples.
In transit
120 For the purposes of sections 113 to 119, if fresh fruits or vegetables are sent or conveyed to Canada in an in bond shipment from a foreign state other than the United States and pass only in transit through the United States, the fresh fruits or vegetables are not considered to have been imported from the United States.
Application for certificate
121 (1) An application for the issuance of a certificate referred to in section 116 or paragraph 117(1)(b) or 118(1)(b) must be made to the Minister in a form approved by the President.
Inspection
(2) The Minister may require an inspection for the purpose of deciding whether to issue the certificate.
Inspection — accessibility
(3) If an inspection is required, the applicant must make the onions, potatoes or apples readily accessible to an inspector at the time of inspection.
SUBDIVISION C
Trade of Fresh Fruits or Vegetables
Prohibition
122 (1) It is prohibited for a person to
- (a) sell any fresh fruits or vegetables that are to be exported or sent or conveyed from one province to another;
- (b) purchase or negotiate the purchase on another person’s behalf of any fresh fruits or vegetables that are to be imported or sent or conveyed from one province to another;
- (c) receive any fresh fruits or vegetables that have been imported or sent or conveyed from one province to another; or
- (d) send or convey from one province to another or import or export any fresh fruits or vegetables.
Exception — persons
(2) Subsection (1) does not apply in respect of
- (a) any person who is a member in good standing of the Fruit and Vegetable Dispute Resolution Corporation, a corporation incorporated under Part 2 of the Canada Not-for-profit Corporations Act, as described in its by-laws;
- (b) any person who only sells fresh fruits or vegetables directly to consumers if that person paid less than $100,000 for the fresh fruits and vegetables that they sold to consumers within the previous 12 months;
- (c) any person who only purchases, sells or negotiates the purchase or sale on another person’s behalf, sends or conveys from one province to another or imports or exports less than one metric ton of fresh fruits and vegetables per day;
- (d) any person who only sells fresh fruits or vegetables that they have grown themselves; or
- (e) a registered charity as defined in subsection 248(1) of the Income Tax Act or a club, society or association described in paragraph 149(1)(l) of that Act.
Exception — nuts, wild fruit and wild vegetables
(3) Subsection (1) does not apply in respect of nuts, wild fruit and wild vegetables.
Damaged or defective fresh fruits or vegetables
123 (1) If a claim is made that all or part of a shipment of fresh fruits or vegetables that is imported or sent or conveyed from one province to another has arrived at its destination in a damaged or defective condition, any person who has a financial interest in that shipment may make a request to the Minister for
- (a) a written report that describes the condition of any damaged or defective fruits or vegetables at the time of the inspection; or
- (b) a written notice of the disposition of any damaged or defective fresh fruits or vegetables that describes the amount of fresh fruit or vegetables that have been disposed of and the place and time of their disposal.
Request for report or notice
(2) The request for a report or notice referred to in subsection (1) must be made to the Minister in a form approved by the President.
Inspection — accessibility
(3) The person who makes the request for a report regarding the condition of fresh fruits or vegetables must make the fresh fruits or vegetables readily accessible to an inspector at the time of inspection.
Disposition of fresh fruit or vegetable
(4) The person who makes the request for a notice regarding the disposition of damaged or defective fresh fruits or vegetables must ensure that their disposition is witnessed by an inspector.
DIVISION 7
Meat Products and Food Animals
SUBDIVISION A
Interpretation
Definitions
124 The following definitions apply in this Division.
on-farm food safety program means a program in respect of food animals that is operated on a farm or at a similar place and under which hazards relating to the safety of meat products that may be derived from those food animals are identified, analyzed and controlled. (programme de salubrité des aliments à la ferme)
specified risk material has the same meaning as in section 6.1 of the Health of Animals Regulations. (matériel à risque spécifié)
split means to cut a carcass lengthwise along the median line. (fendre)
veterinary inspector means a person who is designated as a veterinary inspector under subsection 13(3) of the Canadian Food Inspection Agency Act for the purposes of the Act. (inspecteur vétérinaire)
SUBDIVISION B
Edible Meat Products
Identification of meat products as edible
125 (1) A licence holder may identify a meat product as edible only if
- (a) the food animal from which the meat product is derived, or a sample from the shipment that the food animal is part of, is subjected to an ante-mortem examination under section 138;
- (b) the food animal, other than a game animal, from which the meat product is derived, or a sample from the shipment that the food animal is part of, is subjected to an ante-mortem inspection under section 139;
- (c) the carcass of the food animal from which the meat product is derived is dressed or partially dressed;
- (d) the carcass, its parts and the blood of the food animal from which the meat product is derived are subjected to a post-mortem inspection under subsection 149(1) or a post-mortem examination under subsection 150(1); and
- (e) the meat product is edible and is not contaminated, including that it does not contain any specified risk material.
Certain meat products — additional requirements
(2) Despite (1), a licence holder must not identify as edible any of the following:
- (a) a heart — other than the heart of a domesticated rabbit or of a bird that is not an ostrich, rhea or emu — unless it is opened or inverted and has all blood clots and attached blood vessels removed;
- (b) a liver, unless the gallbladder is removed;
- (c) a gizzard, unless its contents and lining are removed and the gizzard is washed;
- (d) a meat product that contains a urinary bladder, an intestine or any part of a urinary bladder or intestine, unless the bladder, intestine or part is used as a natural casing for the meat product and meets the requirements of section 126; and
- (e) a meat product with an artificial casing, unless the casing is manufactured from a material that is free of any noxious constituent.
Natural casings
126 A urinary bladder, an intestine or any part of a urinary bladder or intestine may be used as a natural casing for an edible meat product if
- (a) the bladder, the intestine or the part of the bladder or intestine have been emptied of their contents and washed, and their mucous lining has been removed;
- (b) in the case of a bladder, it was inverted and placed in brine for at least 12 hours and was subsequently rinsed with water; and
- (c) the casing is clean.
SUBDIVISION C
Humane Treatment
Avoidable death — clarification
127 For greater certainty, a reference to an avoidable death in these Regulations does not include the slaughtering of a food animal in accordance with these Regulations or the humane killing of a food animal.
Avoidable suffering, injury or death
128 A licence holder must handle a food animal at the establishment in a manner that does not cause it avoidable suffering, injury or death and must not subject it to any condition that may cause such suffering, injury or death.
Hitting
129 (1) A licence holder must not hit a food animal with a whip, prod or, except for the purposes of section 141, any other object.
Electric prod
(2) A licence holder must not apply an electric prod to a food animal unless
- (a) it is done for the purpose of causing the animal to move and there is no reasonably practicable alternative to the application of the prod;
- (b) the food animal is a pig or a bovine;
- (c) the prod is applied to the lateral portion of the rear leg muscles between the hock joint and the hip joint;
- (d) the food animal has sufficient space to move forward;
- (e) the food animal’s ability to move is not compromised; and
- (f) the prod is applied in a manner that does not cause the food animal avoidable suffering, injury or death.
Assessing
130 (1) A licence holder must assess whether a food animal is showing signs of suffering or injury on its arrival at the establishment.
Monitoring
(2) After a food animal’s arrival, the licence holder must monitor it on a regular basis, including assessing the conditions to which the food animal is subjected in the establishment that may cause avoidable suffering, injury or death.
Corrective action
(3) If the licence holder determines that conditions exist that may cause avoidable suffering, injury or death to a food animal, the licence holder must immediately take corrective action.
Suffering – immediate measures
(4) If a food animal is showing signs of suffering, the licence holder must immediately
- (a) alleviate its suffering;
- (b) humanely kill it; or
- (c) slaughter it in accordance with these Regulations.
Exception — game animals
(5) This section does not apply in respect of a game animal.
Game animals
131 A licence holder who has direct control over a game animal must
- (a) monitor it on a regular basis, including assessing the conditions to which the game animal is subjected in the establishment that may cause avoidable suffering, injury or death;
- (b) immediately take corrective action if such conditions exist; and
- (c) if the game animal is showing signs of suffering, immediately
- (i) alleviate its suffering,
- (ii) humanely kill it, or
- (iii) slaughter it in accordance with these Regulations.
Segregation and isolation
132 A licence holder must
- (a) segregate food animals of different species;
- (b) segregate a sick or injured food animal with other sick or injured food animals or isolate a sick or injured food animal if, because of its condition, it presents a risk to other food animals or it requires protection from other food animals; and
- (c) isolate a food animal that may cause suffering, injury or death to other food animals because of its nature, temperament, gender, weight, age or any other cause.
Overcrowding
133 A licence holder must provide a food animal with sufficient space to prevent the suffering of, injury to or death of the animal.
Ventilation
134 A licence holder must provide a food animal with sufficient ventilation to prevent the suffering of, injury to or death of the animal.
Handling
135 (1) A licence holder who handles a food animal, including by handling a crate containing a food animal, during any activity they conduct in the establishment, must
- (a) be able to do so, by reason of the person’s competencies and qualifications for the activity, without causing avoidable suffering, injury or death to the food animal; and
- (b) do so in a manner and under circumstances in which the equipment that is used will not cause avoidable suffering, injury or death to the food animal.
Areas of establishment and equipment
(2) A licence holder must, during any activity conducted by the licence holder, use only areas of an establishment and equipment for the handling of a food animal that are designed, constructed and maintained in such a manner that they will not cause avoidable suffering, injury or death to the food animal.
Water and feed
136 (1) A licence holder must provide a food animal — other than a food animal that is confined in a crate — that is unloaded from a conveyance at an establishment with
- (a) water or another source of hydration as soon as it is unloaded; and
- (b) feed, within 24 hours after it is unloaded.
In crate
(2) In the case of a food animal that is confined in a crate, the licence holder must provide the food animal with water or another source of hydration and with feed within 24 hours after it arrives at the establishment.
SUBDIVISION D
Removal and Keeping
Removal
137 (1) A licence holder must notify a veterinary inspector before a food animal is removed from an establishment.
Keeping
(2) A licence holder must notify a veterinary inspector before keeping a food animal in an establishment for more than seven days.
SUBDIVISION E
Ante-mortem Examination and Inspection
Ante-mortem examination
138 (1) Within 24 hours before the slaughter of a food animal and in accordance with the document entitled Ante-mortem Examination and Presentation Procedures for Food Animals, prepared by the Agency and published on its website, as amended from time to time, a licence holder must conduct an ante-mortem examination of the food animal or of a sample from the shipment that the food animal is part of, which must include, in the case an equine or a bird other than one that is a game animal or an ostrich, a rhea or an emu, the examination of the documents referred to in subsection 165(1).
Deviations
(2) If the licence holder, in the course of the ante-mortem examination or at any other time before slaughter, suspects that the food animal shows a deviation from normal behaviour, physiology or appearance, the licence holder must hold it for an inspection by a veterinary inspector, unless otherwise authorized by a veterinary inspector.
Ante-mortem inspection
139 (1) Within 24 hours before the slaughter of a food animal other than a game animal and in accordance with the document entitled Ante-mortem Examination and Presentation Procedures for Food Animals, prepared by the Agency and published on its website, as amended from time to time, a licence holder must, for the purpose of an ante-mortem inspection, present the food animal or a sample from the shipment that the food animal is part of and, in the case an equine or a bird other than an ostrich, a rhea or an emu, the documents referred to in subsection 165(1) to a veterinary inspector or to an inspector under the supervision of a veterinary inspector.
Deviations
(2) If an inspector who is not a veterinary inspector suspects that the food animal shows a deviation from normal behaviour, physiology or appearance, the licence holder must hold it for an inspection by a veterinary inspector.
Condemnation
140 If a veterinary inspector, or an inspector under the supervision of a veterinary inspector, determines after an inspection that any meat product that would be derived from a food animal could not be identified as edible under section 125 and condemns the food animal, a licence holder must
- (a) identify the food animal as inedible;
- (b) segregate the food animal with other condemned food animals or isolate the food animal if, because of its condition, it presents a risk to other food animals or it requires protection from other food animals;
- (c) humanely kill the food animal; and
- (d) identify the carcass and any blood collected from the food animal as inedible.
SUBDIVISION F
Slaughtering and Dressing
Requirement before bleeding
141 Before bleeding a food animal, other than a game animal, a licence holder must render it unconscious in a manner that prevents it from regaining consciousness before death or slaughter it by
- (a) delivering a blow to the head with a mechanical device in a manner that causes an immediate loss of consciousness;
- (b) applying an electrical current in a manner that causes an immediate loss of consciousness; or
- (c) exposing it to a gas or a gas mixture in a manner that causes a rapid loss of consciousness.
Requirement after bleeding starts
142 A licence holder must not cut the carcass of a food animal after bleeding has started if it shows signs of sensibility that may indicate a potential return to consciousness.
Requirement before suspending
143 (1) A licence holder must not suspend a food animal before it is rendered unconscious or slaughtered in accordance with section 141, before it is ritually slaughtered in accordance with section 144 or before it is humanely killed.
Exception — certain birds
(2) Despite subsection (1), a licence holder may suspend a bird, other than an ostrich, rhea or emu, by its legs immediately before it is rendered unconscious or slaughtered in accordance with section 141 or immediately before it is humanely killed.
Ritual slaughter
144 Despite section 141, a licence holder who ritually slaughters a food animal to comply with Judaic or Islamic law must
- (a) restrain the food animal;
- (b) administer one continuous, fluid cut with a knife, without the knife being lifted off the food animal, resulting in the rapid, simultaneous and complete severance of the jugular veins and carotid arteries, in a manner that causes the animal to bleed immediately; and
- (c) rapidly and completely bleed it, to render it unconscious in a manner that prevents it from regaining consciousness before death.
Dressing
145 (1) After a food animal is bled, a licence holder must dress the carcass by doing the following:
- (a) in the case of the carcass of a pig,
- (i) remove the hair, scurf, toenails and developed mammary glands or remove the skin, head, developed mammary glands and feet at the carpal and tarsal joints, and
- (ii) eviscerate and split it;
- (b) in the case of the carcass of a bird,
- (i) remove the feathers and hair or remove the skin,
- (ii) remove the head, uropygial gland and feet at the tarsal joints, and
- (iii) eviscerate it;
- (c) in the case of the carcass of a goat,
- (i) remove the hair, head, toenails and developed mammary glands or remove the skin, head, developed mammary glands and feet at the carpal and tarsal joints, and
- (ii) eviscerate it; and
- (d) in the case of the carcass of any other food animal,
- (i) remove the skin, head, developed mammary glands and feet at the carpal and tarsal joints,
- (ii) eviscerate it, and
- (iii) split it, except in the case of a sheep, calf or domesticated rabbit.
Partial dressing
(2) Despite subsection (1) and at the request of the licence holder, the Minister must authorize the licence holder to partially dress a carcass if
- (a) the licence holder establishes that there is a market for partially dressed carcasses; and
- (b) the licence holder’s procedure for partial dressing is such that the carcass is sufficiently dressed to enable a post-mortem examination or inspection.
Blood clots, bone splinters and extraneous matter
146 A licence holder must remove any blood clot, bone splinter and extraneous matter from the carcass of a food animal and the parts of the carcass, and must identify what was removed as inedible.
Processing of blood
147 A licence holder must process a food animal’s blood in the inedible products area described in subsection 58(2) unless the licence holder
- (a) collects it in a manner that prevents contamination;
- (b) protects it against contamination after it is collected; and
- (c) does not defibrinate it by hand.
Identification and correlation
148 A licence holder must identify the blood of a food animal that is collected to be processed as an edible meat product and the parts of the carcass of the food animal in a manner that enables the correlation of the blood and the parts with the carcass from which they were removed until the completion of the post-mortem inspection or examination.
SUBDIVISION G
Post-mortem Inspection and Examination
Post-mortem inspection
149 (1) A licence holder must, during the course of dressing or partially dressing a carcass, present the carcass, its parts, and any blood of the food animal that is collected to be processed as an edible meat product to a veterinary inspector, or an inspector under the supervision of a veterinary inspector, for a post-mortem inspection.
Deviations
(2) A licence holder, other than a licence holder who is authorized to conduct a post-mortem defect management program, must not, before the post-mortem inspection is completed, remove from the carcass any part that shows a deviation from normal appearance unless authorized to do so by a veterinary inspector.
Deviations — post-mortem defect management program
(3) In the case of a licence holder who is authorized to conduct a post-mortem defect management program, the licence holder must not, before the post-mortem inspection begins, remove from the carcass any part that shows a deviation from normal appearance unless authorized to do so by a veterinary inspector.
Exception — post-mortem examination program
(4) This section does not apply to a licence holder who is authorized to conduct a post-mortem examination program under subsection 160(3).
Post-mortem examination
150 (1) During the course of dressing or partially dressing a carcass, a licence holder who is authorized to conduct a post-mortem examination program under subsection 160(3) must, under the supervision of a veterinary inspector, conduct a post-mortem examination of the carcass, its parts and the blood of the food animal that has been collected to be processed as an edible meat product.
Post-mortem defect management program
(2) During the course of dressing or partially dressing a carcass, a licence holder who is authorized to conduct a post-mortem defect management program under subsection 160(3) must, under the supervision of a veterinary inspector, do the following:
- (a) before the post-mortem inspection begins, conduct a post-mortem screening of the carcass, its parts and the blood of the food animal that has been collected to be processed as an edible meat product; and
- (b) before the post-mortem inspection of the carcass is completed, implement the necessary measures with respect to any defects of the carcass or its parts.
Inspection legend applied before refrigeration
151 In the case of an edible dressed or partially dressed whole carcass or an edible dressed carcass side, other than a carcass or carcass side of a domesticated rabbit or a bird that is not an ostrich, rhea or emu, the inspection legend must be applied after the post-mortem inspection or examination and before refrigeration
- (a) by stamping it directly onto the carcass or carcass side; or
- (b) by applying a label to the carcass or carcass side that is shown prominently and that bears the inspection legend, the date of slaughter of the food animal from which the carcass or carcass side is derived and a code that enables the carcass or carcass side to be correlated with the slaughter of the food animal.
SUBDIVISION H
Inedible Meat Products
Condemnation
152 If a veterinary inspector, or an inspector under the supervision of a veterinary inspector, determines after a post-mortem inspection that any meat product that would be derived from a carcass, any of its parts or the blood of the food animal could not be identified as edible under section 125 and condemns the carcass, its parts or the blood of the food animal, a licence holder must identify any meat product that is derived from the condemned carcass, parts or blood as inedible.
Identification
153 A licence holder must identify as inedible
- (a) the carcass, any of its parts or the blood of a food animal that is rejected by the licence holder who is authorized to conduct a post-mortem examination program or a post-mortem defect management program under subsection 160(3); and
- (b) the carcass of a food animal that dies other than by slaughter in accordance with these Regulations.
Meat products treated as inedible
154 (1) A licence holder may treat as inedible any meat product that
- (a) does not have its movement restricted by an inspector; or
- (b) has its movement restricted by an inspector if the licence holder obtains an inspector’s authorization to move the meat product to the inedible products area described in subsection 58(2).
Identification
(2) The licence holder must identify as inedible any meat product that the licence holder treats as inedible.
Inedible products area
155 (1) When a meat product is condemned or identified as inedible, a licence holder must move it directly to the inedible products area described in subsection 58(2).
Labelling and disposal
(2) The licence holder must take one of the following measures in respect of a meat product that is moved to the inedible products area:
- (a) apply or attach a label to it that indicates its intended use and bears the expression “Not for Use as Human Food” or “ne peut servir à l’alimentation humaine”; or
- (b) dispose of it in accordance with section 66.
Specified risk material
(3) Despite subsection (2), the licence holder must keep a meat product that is a specified risk material, contains a specified risk material or is derived from a specified risk material in a separate area of the inedible products area and must handle and destroy it in accordance with Part I.1 of the Health of Animals Regulations.
SUBDIVISION I
Treatment
Contaminated meat product
156 A licence holder must take one of the following measures in respect of a contaminated meat product:
- (a) subject it to a treatment or process that eliminates the contamination; or
- (b) identify it as inedible.
Trichinella spp. — pork
157 A licence holder may identify as edible a meat product that is derived from a pig and that does not require further preparation before consumption, other than washing or thawing or exposing it to sufficient heat to warm it without cooking it, only if the conditions for identifying the meat product as edible under section 125 are met and
- (a) the pork is subjected to a treatment or process that inactivates Trichinella spp. viable larvae;
- (b) the pork is derived from a carcass that tests negative for the detection of Trichinella spp. larvae using a method that is shown by evidence to be effective; or
- (c) the pig originates from a farm that operates an on-farm food safety program under which the risk of Trichinella spp. infection is negligible.
Trichinella spp. — equine
158 A licence holder may identify as edible a meat product that is derived from an equine only if the conditions for identifying the meat product as edible under section 125 are met and the equine’s carcass tests negative for the detection of Trichinella spp. larvae using a method that is shown by evidence to be effective.
Bovine cysticercosis
159 A licence holder may identify as edible a meat product that is derived from a bovine whose carcass is affected by or shows evidence of bovine cysticercosis only if the conditions for identifying the meat product as edible under section 125 are met and the licence holder has
- (a) removed the parts of the carcass that are affected and identified them as inedible; and
- (b) subjected the remaining parts to a treatment or process that inactivates bovine cysticercosis viable larvae.
SUBDIVISION J
Post-mortem Programs
Application for an authorization
160 (1) The holder of a licence to slaughter may apply in writing to the Minister for an authorization to conduct
- (a) a post-mortem examination program concerning a food animal referred to in the document entitled Fundamentals of the Post-mortem Examination Program, prepared by the Agency and published on its website, as amended from time to time; or
- (b) a post-mortem defect management program concerning a food animal referred to in the document entitled Fundamentals of the Post-mortem Defect Management Program prepared by the Agency and published on its website, as amended from time to time.
Contents of application
(2) The application must include
- (a) the licence holder’s licence number and the address of the establishment where the program will be conducted; and
- (b) a copy of the licence holder’s preventive control plan that meets the requirements of section 89.
Authorization
(3) The Minister must authorize the licence holder to conduct a post-mortem examination program or a post-mortem defect management program if
- (a) the preventive control plan that is included in the licence holder’s application meets the requirements of section 89; and
- (b) the Minister is of the opinion, based on the information that was made available to him or her, that the licence holder is able to conduct the program in compliance with these Regulations.
Grounds for suspension
161 The Minister may suspend a licence holder’s authorization to conduct a post-mortem examination program or a post-mortem defect management program if
- (a) the licence holder does not comply with their post-mortem examination program or post-mortem defect management program;
- (b) the licence holder does not comply with any provision of the Act or of these Regulations; or
- (c) the Minister is of the opinion that a risk of injury to human health may result if the licence holder continues to conduct the program.
Suspension
162 (1) The Minister must not suspend a licence holder’s authorization to conduct a post-mortem examination program or a post-mortem defect management program unless the licence holder
- (a) was provided with an inspection report that sets out the grounds for the suspension and the period within which corrective action must be taken in order to avoid the suspension; and
- (b) failed to take corrective action within that period.
Written notice
(2) The Minister must notify the licence holder in writing of the suspension and the date on which it takes effect.
Risk of injury to human health
163 (1) Despite section 162, if the Minister is of the opinion that a risk of injury to human health may result if the licence holder continues to conduct the post-mortem examination program or post-mortem defect management program, the Minister may suspend the authorization immediately after the licence holder is provided with an inspection report that sets out the grounds for the suspension.
Written notice
(2) The Minister must notify the licence holder in writing that their authorization is suspended and that the suspension takes effect immediately.
Suspension — duration
164 The suspension of an authorization to conduct a post-mortem examination program or post-mortem defect management program must be lifted if the Minister determines that corrective action has been taken.
SUBDIVISION K
Food Animal Information Documents and Document Keeping
Required documents
165 (1) Before slaughtering a food animal that is an equine or a bird, the holder of a licence to slaughter must obtain, from the person who owned or had the possession, care or control of the food animal before its arrival at the establishment where it is intended to be slaughtered, documents that include the following information:
- (a) the name and contact information of the person who owned it and any person who had the possession, care or control of it immediately before its arrival at the establishment;
- (b) the last location where it was raised or kept before its arrival at the establishment, including the address of the location, its code or the number that identifies it;
- (c) the food animal’s identification number or any other information that identifies it;
- (d) the name of any on-farm food safety program under which the food animal was raised or kept;
- (e) in the case of a bird,
- (i) the time at which the first bird of the lot of birds was placed into a crate,
- (ii) the time at which the bird was last provided with access to a source of hydration before being loaded, and
- (iii) the time at which the bird was last provided with access to feed before being loaded;
- (f) a description of any physical or chemical hazard to which the food animal may have been exposed that could cause any meat product that is derived from the food animal to be contaminated;
- (g) in respect of the last 120 days of the life of a bird that has been used for breeding or egg production or in respect of the entire life of any other bird,
- (i) the mortality rate for the flock from which the bird originates,
- (ii) the name of any disease or syndrome that was diagnosed in the flock from which the bird originates and the date on which the flock recovered from the disease or syndrome,
- (iii) the names of any drugs administered to treat a disease or syndrome or administered as an extra-label use and of any vaccines that were administered to the bird, as well as
- (A) their route of administration,
- (B) the first and last date of their administration,
- (C) the dosage that was administered, and
- (D) the withdrawal period or, in the case of an extra-label drug administration, a copy of the prescription that was issued by a veterinarian and an attestation by a competent person or body with respect to the withdrawal period for that administration, and
- (iv) the name of any drug that has been administered in the last 14 days that requires a withdrawal period; and
- (h) in respect of the last 180 days of the life of an equine,
- (i) the name of any disease or syndrome that was diagnosed or a description of any deviation from normal behaviour, physiology or appearance,
- (ii) the names of any drugs and vaccines that were administered to the equine, as well as
- (A) their Drug Identification Numbers, if any,
- (B) their route of administration,
- (C) the last date of their administration,
- (D) the dosage that was administered, and
- (E) the withdrawal period or, in the case of an extra-label drug administration, a copy of the prescription that was issued by a veterinarian and an attestation by a competent person or body with respect to the withdrawal period for that administration, and
- (iii) the use of the equine.
Exception — equines and birds
(2) Despite subsection (1) and subsections 138(1) and 139(1), the licence holder may slaughter the equine or bird without having first obtained the documents referred to in subsection (1) if they notify an inspector before slaughtering the equine or bird and if, subsequent to the slaughter, the meat product that is derived from the equine or bird
- (a) is held by the licence holder until they have obtained those documents, examined them and presented them to a veterinary inspector or to an inspector under the supervision of a veterinary inspector; or
- (b) is identified as inedible.
Exception — game animal, ostrich, rhea and emu
(3) Subsection (1) does not apply in respect of a game animal or an ostrich, rhea or emu.
Retention period of documents
(4) The documents referred to in subsection (1) must be kept for one year after the day on which the food animal arrives at the establishment.
Document keeping
166 (1) The holder of a licence to slaughter must prepare and keep documents that set out the following:
- (a) in the case of a food animal that is slaughtered, the date and time that it was slaughtered and the findings of the ante-mortem examination and, if the licence holder is authorized to conduct a post-mortem examination program or a post-mortem defect management program, the findings of the post-mortem examination or the post-mortem screening and the reason for any condemnation or rejection;
- (b) in the case of a food animal that is found dead at the time of its arrival at the establishment or that dies in the establishment other than by slaughter in accordance with these Regulations,
- (i) the date and time that the food animal was found dead or was humanely killed,
- (ii) its identification number or any other information that identifies it, and
- (iii) the details of its disposal; and
- (c) with respect to the food animals referred to in paragraph (b), the total number per day.
Retention period of documents
(2) The documents referred to in subsection (1) must be kept for one year after the day on which the food animal arrives at the establishment.
SUBDIVISION L
Import and Export
Import
167 The holder of a licence to import may import an edible meat product only if
- (a) the foreign state in which the meat product is manufactured, prepared, stored, packaged or labelled, as the case may be, has, at the time the activity is conducted, an inspection system for meat products that is recognized under Part 7;
- (b) the foreign state from which the meat product is imported has, at the time of the import, an inspection system for meat products that is recognized under Part 7;
- (c) the establishment where the food animal from which the meat product is derived was slaughtered, and any establishment where the meat product was manufactured, processed, treated, preserved, handled, tested, graded, coded, stored, packaged or labelled, have, at the time that the activity is conducted and at the time of the import, a system for manufacturing, processing, treating, preserving, handling, testing, grading, coding, slaughtering, storing, packaging or labelling, as the case may be, that is recognized under Part 7; and
- (d) the licence holder provides an inspector with an official document issued by the foreign state, in a form approved by the President, that states that the meat product meets the requirements that are set out in the Act and these Regulations.
Export
168 (1) A licence holder may export an edible meat product only if
- (a) the licence holder provides an inspector with a document that substantiates that the requirements of the foreign state to which it is intended to be exported are met in respect of that meat product; and
- (b) a certificate or other document is issued under section 48 of the Act in respect of that meat product.
Exception — removal notice
(2) Subsection (1) does not apply in respect of a meat product that is subject to a notice ordering its removal from Canada under subsection 32(1) of the Act.
PART 7
Recognition of Foreign Systems
Exception — shellfish
169 This Part does not apply in respect of the adductor muscles of scallops and the meat of geoducks.
Application for recognition of inspection system
170 (1) A foreign state may make an application to the Minister in writing for the recognition of its inspection system for meat products or live or raw shellfish.
Contents of application
(2) The application must include the following information:
- (a) in the case of an inspection system for meat products,
- (i) an indication of the species of birds or mammals and a description of the meat products to which the system applies, and
- (ii) the approximate number of establishments where the manufacturing, preparing, storing, packaging or labelling of meat products that are intended to be exported to Canada would be conducted and an indication of the activities that would be conducted in those establishments;
- (b) in the case of an inspection system for live or raw shellfish, an indication of the species and the growing and harvesting areas to which the system applies;
- (c) the volume of meat products or live or raw shellfish to which the system applies that is anticipated to be exported to Canada per year; and
- (d) the items set out in paragraph (3)(a) or (b), as the case may be; and
- (e) the name, title and signature of the authorized representative of the foreign state who makes the application.
Recognition by Minister
(3) The Minister must recognize the inspection system in respect of which the application is made if the system provides at least the same level of protection as that provided by the provisions of the Act and these Regulations, taking into account the following:
- (a) in the case of an inspection system for meat products,
- (i) any applicable legislative framework, controls and procedures,
- (ii) the organizational structure of the authority that is responsible for the system,
- (iii) the implementation of the system,
- (iv) the resources that support the objectives of the system,
- (v) the humane treatment of the food animals that are intended to be slaughtered,
- (vi) the chemical residue monitoring and microbiological monitoring of the meat products,
- (vii) the certification process for the export of the meat products, and
- (viii) any other relevant information; and
- (b) in the case of an inspection system for live or raw shellfish,
- (i) any applicable legislative framework, controls and procedures,
- (ii) the organizational structure of the authority that is responsible for the system,
- (iii) the implementation of the system,
- (iv) the resources that support the objectives of the system,
- (v) the chemical and microbiological monitoring of the shellfish, including monitoring for biotoxins,
- (vi) the monitoring of the waters in the growing and harvesting areas to assess their suitability for their intended purpose, and
- (vii) any other relevant information.
Application for recognition of system
171 (1) If a foreign state’s inspection system for meat products is recognized, the foreign state may make an application to the Minister in writing for the recognition of the system of manufacturing, processing, treating, preserving, handling, testing, grading, coding, slaughtering, storing, packaging or labelling that is used in an establishment and that is subject to that inspection system.
Contents of application
(2) The application must include the following information:
- (a) the name of the person who conducts any relevant activities and the establishment’s address;
- (b) the establishment’s registration number, or another identification number for the establishment, that is provided by the foreign state;
- (c) a statement that identifies the system in respect of which the application is made;
- (d) a declaration by the authorized representative of the foreign state who makes the application that states that the system in respect of which the application is made is subject to the foreign state’s recognized inspection system and meets the requirements of that inspection system that apply to the conduct of those activities in respect of meat products that are intended to be exported to Canada; and
- (e) the name, title and signature of the authorized representative of the foreign state who makes the application.
Recognition by Minister
(3) The Minister must recognize a system of manufacturing, processing, treating, preserving, handling, testing, grading, coding, slaughtering, storing, packaging or labelling in respect of which an application is made if
- (a) the foreign state’s inspection system for the relevant meat products is recognized under subsection 170(3); and
- (b) the system of manufacturing, processing, treating, preserving, handling, testing, grading, coding, slaughtering, storing, packaging or labelling is subject to the inspection system referred to in paragraph (a) and meets the requirements of that inspection system that apply to those activities in respect of meat products that are intended to be exported to Canada.
Suspension of recognition — inspection system
172 (1) The Minister must suspend the recognition of a foreign state’s inspection system that is referred to in section 170 if
- (a) the foreign state fails to notify the Minister in writing, as soon as feasible, of any changes that it has made to the system or to the legislation governing the system; or
- (b) the system no longer provides at least the same level of protection as that provided by the provisions of the Act and these Regulations.
Suspension of recognition — system used in establishment
(2) The Minister must suspend the recognition of a system of manufacturing, processing, treating, preserving, handling, testing, grading, coding, slaughtering, storing, packaging or labelling that is referred to in section 171 if
- (a) the system no longer meets the foreign state’s requirements that apply to those activities;
- (b) a shipment of meat products that have been manufactured, processed, treated, preserved, handled, tested, graded, coded, stored, packaged or labelled using the system is determined to contravene a provision of the Act, these Regulations, the Food and Drugs Act or the Food and Drug Regulations, and
- (i) during the six-month period immediately prior to that shipment, there have been two other shipments of meat products that have been manufactured, processed, treated, preserved, handled, tested, graded, coded, stored, packaged or labelled using the system in respect of which such a determination of non-compliance has been made, or
- (ii) among the four most recent shipments, prior to that shipment, of meat products that have been manufactured, processed, treated, preserved, handled, tested, graded, coded, stored, packaged or labelled using the system, two shipments have been determined to be non-compliant;
- (c) the system is no longer subject to the foreign state’s inspection system; or
- (d) the recognition of the foreign state’s inspection system to which the system is subject has been suspended.
Notice
(3) The Minister must notify the foreign state in writing of the suspension under subsection (1) or (2), the grounds for the suspension and the date on which it takes effect.
Effective date
(4) The suspension takes effect on the day on which the notice is issued.
Reinstatement of recognition
(5) The Minister must notify the foreign state in writing that the recognition is no longer suspended if
- (a) in the case of a suspension under subsection (1) or under paragraph (2)(a), (c) or (d), the circumstances that gave rise to a suspension have been remedied; or
- (b) in the case of a suspension under paragraph (2)(b), the establishment has taken corrective action.
Cancellation of recognition — inspection system
173 (1) The Minister must cancel the recognition of a foreign state’s inspection system referred to in section 170 if
- (a) no meat products or live or raw shellfish to which the system applies have been exported to Canada from the foreign state in the previous five years;
- (b) the circumstances that gave rise to a suspension have not been remedied within 12 months after the day on which the recognition was suspended; or
- (c) the foreign state requests the cancellation in writing.
Cancellation of recognition — system used in establishment
(2) The Minister must cancel the recognition of a system of manufacturing, processing, treating, preserving, handling, testing, grading, coding, slaughtering, storing, packaging or labelling referred to in section 171 if
- (a) the recognition of the inspection system to which it is subject has been cancelled;
- (b) no meat products that were manufactured, processed, treated, preserved, handled, tested, graded, coded, stored, packaged or labelled using the system have been exported to Canada from the foreign state in the previous five years;
- (c) circumstances that gave rise to a suspension have not been remedied within 12 months after the day on which the recognition was suspended; or
- (d) the foreign state in which the establishment is located requests the cancellation in writing.
Notice
(3) The Minister must notify the foreign state in writing of the cancellation under subsection (1) or (2), the grounds for the cancellation and the date on which it takes effect.
Effective date
(4) The cancellation takes effect on the day on which the notice is issued.
PART 8
Ministerial Exemptions
Application for exemption — test marketing or shortage in supply
174 (1) Any person may apply, in a form approved by the President, for an exemption from the application of a provision of the Act or these Regulations for the purpose of test marketing a food or of alleviating a shortage in Canada in the available supply of a food that is manufactured, processed or produced in Canada.
Exemption
(2) The Minister may, in writing, grant the exemption if
- (a) the information submitted in the application for the exemption is complete, truthful and not misleading;
- (b) the food in respect of which the application for the exemption is made meets the requirements that are set out in paragraphs 8(1)(a) to (d);
- (c) the Minister is of the opinion that no risk of injury to human health will result;
- (d) in the case of an application for an exemption for the purpose of test marketing a food, the Minister is of the opinion that the exemption will not
- (i) confuse or mislead the public, or
- (ii) disrupt the normal trading patterns of the industry or the normal food pricing patterns; and
- (e) in the case of an application for an exemption for the purpose of alleviating a shortage in Canada in the available supply of a food that is manufactured, processed or produced in Canada, the exemption is necessary to alleviate that shortage.
Application for exemption — inspection legend
175 (1) The holder of a licence to slaughter food animals and to process meat products may apply, in a form approved by the President, for an exemption from the application of section 151 in respect of a carcass or carcass side.
Exemption
(2) The Minister may, in writing, grant the exemption if
- (a) the information submitted in the application for the exemption is complete, truthful and not misleading;
- (b) the licence holder has established procedures
- (i) for processing the carcass or carcass side within the establishment where the food animal was slaughtered, and
- (ii) for identifying the carcass or carcass side to allow it to be linked with any edible meat products derived from it and for the keeping of documents to allow for this linkage;
- (c) the Minister is of the opinion that no risk of injury to human health will result; and
- (d) the Minister is of the opinion that no risk of harm to interprovincial trade or export will result.
Conditions
(3) The exemption granted under subsection (2) is subject to the following conditions:
- (a) the carcass or carcass side must be processed within the establishment where the food animal was slaughtered; and
- (b) the carcass or carcass side must be identified to allow it to be linked with any edible meat products derived from it and documents must be kept to allow for this linkage.
Additional conditions
176 The Minister may, at any time, make any exemption referred to in subsection 174(2) or 175(2) subject to conditions.
Period of validity
177 An exemption referred to in subsection 174(2) or 175(2) is valid until the expiry date that is specified in the exemption or, if no date is specified, until the end of the period that is two years after the day on which the exemption is granted.
Cancellation
178 The Minister may cancel an exemption if
- (a) the Minister is of the opinion that to not cancel the exemption may result in a risk of injury to human health;
- (b) in the case of an exemption referred to in subsection 175(2), the Minister is of the opinion that to not cancel the exemption may result in a risk of harm to interprovincial trade or export; or
- (c) the person who has been granted the exemption does not comply with any condition to which the exemption is subject or any provision of the Act or these Regulations other than a provision in respect of which the exemption is granted.
PART 9
Inspection Legends
Definition of inspection mark in Act
179 The inspection legends that are set out in Figures 1 and 2 of Schedule 2 are prescribed for the purposes of the definition inspection mark in section 2 of the Act.
Edible meat products — Figure 1 of Schedule 2
180 (1) A licence holder or an inspector is authorized to apply the inspection legend that is set out in Figure 1 of Schedule 2 to, and use it in connection with, an edible meat product, whether prepackaged or not, if the following conditions are met:
- (a) the meat product was manufactured, processed, treated, preserved, packaged or labelled by the licence holder in accordance with the provisions of the Act and these Regulations;
- (b) in the case where any meat product that it contains was manufactured, processed, treated, preserved, packaged or labelled in Canada, that activity was conducted by a licence holder in accordance with the provisions of the Act and these Regulations;
- (c) in the case where the meat product is a livestock carcass or poultry carcass that was graded in Canada, or is derived from such a carcass, that activity was conducted by a grader in accordance with these Regulations;
- (d) in the case where any meat product that it contains was imported, it was imported by a licence holder in accordance with the provisions of the Act and these Regulations;
- (e) in the case where the meat product, or any meat product that it contains, is derived from food animals that were slaughtered in Canada, the food animals were slaughtered by a licence holder in accordance with the provisions of the Act and these Regulations;
- (f) the meat product complies with the standards that are set out in Volume 7 of the Standards of Identity Document and meets the requirements that are set out in paragraphs 8(1)(a) to (d) and in Division 7 of Part 6; and
- (g) the inspection legend is applied or used in an establishment that is identified in the licence holder’s licence, unless the meat product is intended to be exported and the inspection legend is applied to the conveyance or used in connection with the conveyance in which the meat product is exported.
Edible meat products — Figure 2 of Schedule 2
(2) A licence holder or an inspector is authorized to apply the inspection legend that is set out in Figure 2 of Schedule 2 to, and use it in connection with, an edible prepackaged meat product if the conditions set out in subsection (1) are met and the container of the meat product
- (a) is a hermetically sealed package that is labelled in a legible and permanent manner so as to make it possible to identify the establishment that is identified in the licence holder’s licence;
- (b) is a casing or bag that is closed by a clip, if the number identifying the licence holder’s establishment is legibly engraved on the clip and is visible when the clip is closed; or
- (c) bears the number identifying the licence holder’s establishment on the label except if it is shown on any part of the label that is applied or attached to the bottom of the container.
Exception — export under section 16
(3) In the case of the export of a meat product under subsection 16(1), the requirements of subsections (1) and (2) must be met except any requirement that is set out in paragraph (1)(f) in the case where it is the unmet requirement referred to in subsection 16(1).
Exception — dressed beef carcass side
(4) Despite paragraph (1)(f), a licence holder is authorized to apply, after the post-mortem inspection or examination and before refrigeration, in the manner set out in paragraph 151(a) or (b), the inspection legend set out in Figure 1 of Schedule 2 to a dressed beef carcass side containing dorsal root ganglia if the carcass side has been clearly marked to identify it as containing dorsal root ganglia. The dorsal root ganglia must be removed from the carcass side before any meat product derived from it is identified as edible.
Processed egg products
181 A licence holder or an inspector is authorized to apply the inspection legend that is set out in Figure 1 of Schedule 2 to, and use it in connection with, a prepackaged processed egg product if the following conditions are met:
- (a) the processed egg product was manufactured, processed, treated, preserved, packaged or labelled by the licence holder in accordance with the provisions of the Act and these Regulations;
- (b) if the processed egg product or any processed egg product that it contains was manufactured, processed, treated, preserved, packaged or labelled in Canada, that activity was conducted by a licence holder in accordance with the provisions of the Act and these Regulations;
- (c) if the eggs from which the processed egg product is derived were processed, treated, preserved, graded, packaged or labelled in Canada, that activity was conducted by a licence holder in accordance with the provisions of the Act and these Regulations;
- (d) the processed egg product complies with the standards that are set out in Volume 2 of the Standards of Identity Document and meets the requirements that are set out in paragraphs 8(1)(a) to (d) and in Division 4 of Part 6; and
- (e) the inspection legend is applied or used in an establishment that is identified in the licence holder’s licence.
Fish
182 A licence holder or an inspector is authorized to apply the inspection legend that is set out in Figure 1 or 2 of Schedule 2 to, and use it in connection with, prepackaged fish if the following conditions are met:
- (a) the fish was manufactured, processed, treated or preserved by the licence holder in accordance with the provisions of the Act and these Regulations;
- (b) if the fish or any fish that it contains was manufactured, processed, treated, preserved, graded, packaged or labelled in Canada, that activity was conducted by a licence holder in accordance with the provisions of the Act and these Regulations; and
- (c) the fish complies with the standards that are set out set out in Volume 3 of the Standards of Identity Document and meets the requirements that are set out in paragraphs 8(1)(a) to (d) and in Division 5 of Part 6.
Number identifying establishment
183 A licence holder or an inspector who applies or uses the inspection legend that is set out in Figure 1 of Schedule 2 must replace the numbers “00” with the number identifying the licence holder’s establishment.
Authorized use
184 (1) The following persons are authorized to use the inspection legends that are set out in Figures 1 and 2 of Schedule 2:
- (a) printers of labels, other than printers of official export labels, and manufacturers of packages, if the labels and packages that bear the inspection legend are provided to any person who is authorized under any of sections 180 to 182 to apply and use the inspection legend;
- (b) printers of official export labels, if the labels that bear the inspection legend are provided to an inspector;
- (c) publishers of documents on the subject of the inspection of meat products, processed egg products or fish;
- (d) publishers of documents that advertise meat products, processed egg products or fish; and
- (e) manufacturers of stamps, if the stamps that bear the inspection legend are provided to any person who is authorized under any of sections 180 to 182 to apply and use the inspection legend.
Advertising and sale
(2) Any person who is authorized under subsection (1) to use an inspection legend is also authorized to advertise the labels, packages, documents and stamps, as the case may be, which bear the inspection legend and to sell them.
Advertising and sale of certain foods
185 Any person is authorized to advertise and sell a meat product, prepackaged processed egg product or prepackaged fish to which an inspection legend has been applied or in connection with which an inspection legend is used, if the inspection legend was applied or is used in accordance with these Regulations. This includes using an inspection legend set out in Figures 1 and 2 of Schedule 2 for advertising the meat product, prepackaged processed egg product or prepackaged fish.
PART 10
Packaging
DIVISION 1
General
Requirements for packages
186 A prepackaged food that is sent or conveyed from one province to another or that is imported or exported must meet the following requirements:
- (a) its package
- (i) must be suitable for its intended use and appropriate for the food,
- (ii) must be capable of protecting the food against moisture, loss, damage, contamination and deterioration during normal handling, storing and conveying,
- (iii) must be clean and in a sanitary condition,
- (iv) must be of sound construction,
- (v) must be free from odours that might affect the food,
- (vi) must not impart any undesirable substance to the food,
- (vii) must not have a design or mark, or be of a colour, that enhances the appearance of the food with respect to its quality or composition, and
- (viii) must be new, in the case of
- (A) a liner that is used in connection with a processed egg product,
- (B) a package of a processed egg product, if the package is made of corrugated fibreboard,
- (C) an egg carton of eggs that are graded in accordance with these Regulations, and
- (D) a tray of eggs that are graded Canada A or Canada B that is made of molded pulp;
- (b) in the case of a processed egg product, its package must, if it has previously been used and is not constructed of corrosion-resistant material, be lined with a sanitary plastic or equivalent liner;
- (c) in the case of eggs that are graded in accordance with these Regulations, its package must, if it is a plastic tray that has previously been used, be sanitized and dry before reuse; and
- (d) in the case of eggs that are graded Canada A or Canada B, its package must not have previously been used to package ungraded eggs or eggs that are graded Canada Nest Run.
DIVISION 2
Standard Container Sizes
Application
187 The requirements of this Division apply in respect of any food that is sent or conveyed from one province to another, imported, or, except if it is set out in column 1 of Table 1 of Schedule 3 or in column 1 of items 5 to 10 of Table 2 of that Schedule, exported.
Table 1 of Schedule 3 — weight or volume requirements
188 (1) The container of a consumer prepackaged food that is set out in column 1 of Table 1 of Schedule 3 must be of a size that corresponds to a net quantity by weight or by volume that is set out in column 2 or 3.
Exception
(2) Subsection (1) does not apply in respect of a consumer prepackaged food that is
- (a) manufactured, prepared, produced, packaged or labelled for use by commercial or industrial enterprises or institutions without being sold by them as consumer prepackaged foods;
- (b) manufactured, prepared, produced, packaged or labelled only for sale to or by a duty free shop; or
- (c) distributed to one or more persons for no consideration.
Table 2 of Schedule 3 — weight requirements
189 (1) Subject to subsections (3) and (4) and section 192, the container of a consumer prepackaged food that is set out in column 1 of Table 2 of Schedule 3 must be of a size that corresponds to a net quantity by weight that is set out in column 2.
Table 3 of Schedule 3 — weight requirements
(2) Subject to section 192, the container of a prepackaged food that is set out in column 1 of Table 3 of Schedule 3 must be of a size that corresponds to a net quantity by weight that is set out in column 2.
Exception
(3) Subsection (1) does not apply in respect of a consumer prepackaged food that is set out in column 1 of items 2 to 4 of Table 2 of Schedule 3 if
- (a) in the case of a catch-weight food, a label that bears the net weight for retail sale is applied or attached to the food;
- (b) its container is a hermetically sealed package; or
- (c) it has a net quantity that is greater than 1 kg.
Exception — volume capacity
(4) The container of a consumer prepackaged food that is set out in column 1 of items 5 to 10 of Table 2 of Schedule 3 may have any volume capacity that is set out in Table 7 of that Schedule, in the case of metric containers, or Table 8 of that Schedule, in the case of imperial containers.
Table 4 of Schedule 3 — volume and dimension requirements
190 Subject to section 192, the container of a prepackaged food that is set out in column 1 of Table 4 of Schedule 3 must be of a size that corresponds to a net quantity by volume that is set out in column 2 or 3 and must have the dimensions that are set out in column 4 or 5 for that net quantity.
Table 5 of Schedule 3 — volume and dimension requirements
191 (1) Subject to section 192, the container of a prepackaged food for which a grade is prescribed by these Regulations and that is set out in column 1 of Table 5 of Schedule 3 must, if the container is a hermetically sealed package, be of a size that corresponds to a net quantity by volume that is set out in column 2 or 3 and, if it is a metal container, it must also have the dimensions that are set out in column 4 or 5 for that net quantity.
Table 6 of Schedule 3 — volume and dimension requirements
(2) Subject to section 192, the container of a prepackaged food for which no grade is prescribed by these Regulations and that is set out in column 1 of Table 6 of Schedule 3 must, if the container is a hermetically sealed package, be of a size that corresponds to a net quantity by volume that is set out in column 2 or 3 and, if it is a metal container, it must also have the dimensions that are set out in column 4 or 5 for that net quantity.
Exception
192 The container of a prepackaged food that is set out in column 1 of items 2 to 11 of Table 3 of Schedule 3 or in column 1 of Table 4, 5 or 6 of that Schedule may be of a size that is greater than the sizes that are required by sections 189 to 191 if
- (a) the container holds a net quantity of food that is
- (i) not more than 20 kg, in the case of a food that is packaged by weight, or
- (ii) not more than 20 L, in the case of a food that is packaged by volume; and
- (b) the declared net quantity of food that is shown on the label is a whole number multiple of
- (i) 500 g, in the case of a food that is packaged by weight, or
- (ii) 500 mL, in the case of a food that is packaged by volume.
Certain prepackaged fresh fruits or vegetables
193 (1) The container of prepackaged fresh fruits or vegetables, other than consumer prepackaged fresh fruits or vegetables that are set out in column 1 of items 5 to 10 of Table 2 of Schedule 3, for which a grade is prescribed by these Regulations must have a capacity that is not greater than
- (a) 200 kg net weight, in the case of apples, pears, peaches and apricots; and
- (b) 50 kg net weight, in the case of any other fresh fruits or vegetables.
Exception
(2) Subsection (1) does not apply in respect of fresh fruits or vegetables that are exported.
DIVISION 3
Standards of Fill for Processed Fruit or Vegetable Products
Application
194 The requirements of this Division apply in respect of any foods that are sent or conveyed from one province to another, imported or exported.
Frozen processed fruit or vegetable products
195 At least 90% of the volume capacity of the package of a frozen processed fruit or vegetable product must be filled with the product.
Non-frozen processed fruit or vegetable products
196 The package of a processed fruit or vegetable product that is not frozen
- (a) must be filled with as much of the product as its preparation allows; and
- (b) must not contain more syrup, brine, water or other liquid packaging medium than is necessary for the processing of the product.
Hermetically sealed packages
197 Despite section 196, a processed fruit or vegetable product that is in a hermetically sealed package must meet the requirements for minimum drained weight and average drained weight that are set out in the document entitled Minimum Drained Weights and Average Drained Weights for Processed Fruit or Vegetable Products in a Hermetically Sealed Package, prepared by the Agency and published on its website, as amended from time to time.
PART 11
Labelling
DIVISION 1
General
SUBDIVISION A
Interpretation
Definitions
198 The following definitions apply in this Part.
Canadian unit means a unit of measurement that is set out in Schedule II to the Weights and Measures Act. (unité canadienne)
metric unit means a unit of measurement that is set out in Schedule I to the Weights and Measures Act. (unité métrique)
SUBDIVISION B
Subsection 6(1) of Act
False, misleading or deceptive labelling
199 (1) For the purposes of subsection 6(1) of the Act, labelling a food in a manner that is false, misleading or deceptive or is likely to create an erroneous impression includes labelling a food with
- (a) any representation in which expressions, words, figures, depictions or symbols are used, arranged or shown in a manner that may reasonably be considered to qualify the declared net quantity of a consumer prepackaged food or that is likely to deceive with respect to the net quantity of a consumer prepackaged food; or
- (b) any expression, word, figure, depiction or symbol that may reasonably be considered to imply that a consumer prepackaged food contains any matter that it does not in fact contain or that it does not contain any matter that it does in fact contain.
False, misleading or deceptive selling, importing and advertising
(2) For the purposes of subsection 6(1) of the Act, selling, importing or advertising a food in a manner that is false, misleading or deceptive or is likely to create an erroneous impression includes selling, importing or advertising a consumer prepackaged food that is labelled in the manner set out in paragraph (1)(a) or (b).
Declaration of net quantity — labelling
(3) For the purposes of subsection 6(1) of the Act, labelling a consumer prepackaged food with a declaration of net quantity does not constitute labelling a food in a manner that is false, misleading or deceptive if
- (a) the declaration meets any requirements under the Act; and
- (b) the actual net quantity of the food is, subject to the tolerance provided under subsection (5), not less than its declared net quantity.
Declaration of net quantity — selling, importing or advertising
(4) For the purposes of subsection 6(1) of the Act, selling, importing or advertising a consumer prepackaged food that is labelled with a declaration of net quantity does not constitute selling, importing or advertising a food in a manner that is false, misleading or deceptive if the conditions set out in paragraphs (3)(a) and (b) are met.
Tolerance — paragraph (3)(b)
(5) For the purposes of paragraph (3)(b), the tolerance for a declared net quantity that is set out in column 1 of the applicable table in Schedule 4 is the tolerance that is set out in column 2 or 3 for that net quantity.
Inspection — net quantity
200 (1) An inspection of a lot of food, each unit of which purports to contain the same net quantity, by an inspector for the purpose of determining whether the lot meets the conditions of paragraphs 199(3)(a) and (b) must be conducted by collecting and examining a sample from the lot.
Number of units collected — Schedule 5
(2) Subject to subsection (3), if a lot contains the number of units set out in column 1 of Part 1 of Schedule 5, the inspector must collect from the lot at least the number of units set out in column 2 and the units collected constitute the sample referred to in subsection (1).
Destruction of units
(3) If, for the purpose of determining the net quantity, other than for establishing the weight of the container, it is necessary to destroy a certain number of units in the lot, an inspector must not collect a number of units intended to be destroyed that is more than 10% of the total number of units in the lot or less than one unit, and the units collected constitute the sample referred to in subsection (1).
Determination of inspector
(4) The lot from which a sample was collected and examined by an inspector does not meet the conditions of paragraphs 199(3)(a) and (b) if the inspector determines that
- (a) the weighted average quantity of the units in the sample, as determined by the formula set out in Part 2 of Schedule 5, is less than the declared net quantity;
- (b) the number of units in the sample that contain less than the declared net quantity by more than the prescribed tolerance set out in Schedule 4 for that quantity is equal to or greater than the number set out in column 2 of Part 4 of Schedule 5 for the sample size set out in column 1 of that Part; or
- (c) two or more units in the sample contain less than the declared net quantity by more than twice the prescribed tolerance set out in Schedule 4 for that quantity.
Liquid consumer prepackaged food
(5) In the case of an inspection of a consumer prepackaged food that consists of a liquid, the net quantity of the food must be determined on the basis of the assumption that the liquid is at a temperature of 20°C.
Frozen liquid consumer prepackaged food
(6) In the case of an inspection of a consumer prepackaged food that consists of a frozen liquid food and that is normally sold and consumed in a frozen state, the net quantity of the food must be determined when the food is in a frozen state.
SUBDIVISION C
Standards Prescribed for Food
Common names
201 A food, whether prepackaged or not, that is sent or conveyed from one province to another or that is imported or exported, and whose label bears a common name printed in boldface type, but not in italics, in the Standards of Identity Document must meet any standard that applies in respect of that common name.
Icewine
202 It is prohibited for a person to sell a consumer prepackaged food whose label bears the word or expression “icewine” or “ice wine” or “ice-wine” or “vin de glace” or any similar word or expression, or any abbreviation of, symbol for or phonetic rendering of any of those words or expressions, unless the food meets the standard set out in Volume 8 of the Standards of Identity Document.
SUBDIVISION D
Information
Compliance with requirements of this Part
203 (1) An item of information that is shown on the label of a prepackaged food must meet any requirement of this Part that applies to that item of information, even if these Regulations do not require the food to be labelled with that item of information.
Application
(2) Subsection (1) applies in respect of
- (a) in the case of an item of information referred to in Division 2, any prepackaged food that is sold in Canada and any prepackaged food that is sent or conveyed from one province to another or that is imported; and
- (b) in the case of an item of information referred to in these Regulations, other than in Division 2, any food that is sent or conveyed from one province to another or that is imported or exported.
Application — paragraphs 218(1)(a) and (b)
(3) In the case of an item of information referred to in paragraphs 218(1)(a) and (b), subsection (1) also applies in respect of any dairy products, eggs, processed egg products, fish, fresh fruits or vegetables, processed fruit or vegetable products, honey, maple products or meat products that are prepackaged and exported.
Use of word “classifié”
204 If a provision of these Regulations requires the word “classifié” to be shown on a label, the word “classé” may be used in its place.
SUBDIVISION E
Official Languages
Prepackaged food
205 (1) Any information that is required by these Regulations to be shown on the label of a prepackaged food, other than a consumer prepackaged food, and that is not information referred to in subsection (3) must be shown on the label in at least one official language.
Exception — words or expressions in quotation marks
(2) For the purposes of subsection 48(2), paragraph 254(b), subsections 256(1) and 257(1), paragraph 286(c) and subsection 333(2), both an English word or expression that appears in quotation marks and a French word or expression that appears in quotation marks must be shown on the label of a prepackaged food except in the case where the label of that food is authorized to show information in only one official language under subsections B.01.012(2) to (10) of the Food and Drug Regulations.
Exception — both official languages
(3) The label of a prepackaged food must bear, in both official languages,
- (a) the size designation of eggs that are graded Canada A referred to in section 316; and
- (b) the colour class of maple syrup that is graded Canada Grade A or graded Grade A referred to in section 325.
Consumer prepackaged food
206 (1) The information that is required by these Regulations to be shown on the label of a consumer prepackaged food must be shown in both official languages, except in the case where the label of that food is authorized to show information in only one official language under subsections B.01.012(2) to (10) of the Food and Drug Regulations.
Words or expressions in quotation marks
(2) A provision of these Regulations — other than paragraphs 110(a) and 111(a) — that requires a consumer prepackaged food to be labelled with words or expressions that appear in quotation marks must be read to require both an English word or expression and a French word or expression to be shown on the label of the food, except in the case where the label of that food is authorized to show information in only one official language under subsections B.01.012(2) to (10) of the Food and Drug Regulations.
Exception — only one official language
(3) Despite subsection (1), the grade name may be shown on the label of consumer prepackaged fish in only one official language.
Modifications
207 For the purposes of subsections 205(2) and 206(1) and (2),
- (a) the expression “principal display panel” in subsections B.01.012(8) and (10) of the Food and Drug Regulations has the same meaning as in section 1 of these Regulations;
- (b) a reference to “these Regulations” in subsections B.01.012(2), (3), (7) and (8) of the Food and Drug Regulations must be read as a reference to “Part 11 of the Safe Food for Canadians Regulations”; and
- (c) a reference to “manufactured, processed, produced or packaged”, “manufactured, processed or packaged” or “manufactured, processed, produced or packaged for resale” in the definitions local food and specialty food in subsection B.01.012(1) of the Food and Drug Regulations and in subsection B.01.012(9) of those Regulations must be read as a reference to “manufactured, processed, treated, preserved, produced or packaged”.
SUBDIVISION F
Legibility and Type Size
Legibility
208 Any information that a label is required by these Regulations to bear must be clearly and prominently shown and readily discernible and legible to the purchaser under the customary conditions of purchase and use.
Upper or lower case
209 If a word or expression that appears in quotation marks in these Regulations is required to be shown on a label, it may, unless otherwise provided, be shown in upper or lower case, or both, so long as it meets the legibility and character height requirements of these Regulations.
Type size
210 (1) This section applies unless another provision of this Part specifies a character height for a particular item of information.
Consumer prepackaged food
(2) The information that a label of a consumer prepackaged food is required by this Part to bear must be shown in characters that are at least 1.6 mm in height.
Exception
(3) That information, other than the declaration of net quantity, may be shown in characters that are at least 0.8 mm in height if
- (a) the information that a label is required by Division 2 to bear is shown on the principal display panel; and
- (b) the area of the principal display surface is 10 cm2 or less.
Measurement of type size
211 The height of the characters in words shown on a label must be determined by measuring
- (a) the height of an upper case letter, if the words are shown in upper case only; and
- (b) the height of the lower case letter “o”, if the words are shown in lower case or in both upper and lower case.
DIVISION 2
Requirements Applicable to Prepackaged Food
SUBDIVISION A
Application of this Division
Sale, interprovincial trade or import
212 (1) The requirements of this Division apply in respect of any prepackaged food that is sold in Canada, sent or conveyed from one province to another or imported.
Export
(2) Section 217, paragraphs 218(1)(a) and (b) and section 225 also apply in respect of any dairy products, eggs, processed egg products, fish, fresh fruits or vegetables, processed fruit or vegetable products, honey, maple products or meat products that are prepackaged and exported.
Exception — sections 214 and 217
213 Sections 214 and 217 do not apply in respect of any prepackaged foods that are
- (a) confections that are sold individually, commonly known as one-bite confections;
- (b) fresh fruits or vegetables that are packaged in a wrapper, or a confining band, that is less than 13 mm in width; or
- (c) fresh fruits or vegetables that are packaged in a protective wrapper, or a protective bag, that is transparent and on which no information is shown other than a price, bar code, number code, environmental statement or product treatment symbol.
SUBDIVISION B
Sale and Advertisement
Sale — prepackaged food
214 It is prohibited for a person to sell a prepackaged food unless a label that meets the requirements of Divisions 1 and 2 is applied or attached to the prepackaged food in the manner set out in these Regulations.
Advertising — consumer prepackaged food
215 It is prohibited for a person to advertise a consumer prepackaged food unless a label is applied or attached to the food in the manner set out in Divisions 1 and 2.
Representations relating to net quantity
216 It is prohibited for a person, in advertising a consumer prepackaged food, to make any representation with respect to the net quantity of the food except in the manner set out in Divisions 1 and 2.
SUBDIVISION C
Label Required
Prepackaged food
217 A prepackaged food must have a label that meets the requirements of these Regulations applied or attached to it in the manner set out in these Regulations.
SUBDIVISION D
Information
Prepackaged Foods
Prepackaged foods — label
218 (1) Unless otherwise provided by this Part, a label that is applied or attached to a prepackaged food must bear
- (a) the common name of the food, shown on the principal display panel;
- (b) the name and principal place of business of the person by or for whom the food was manufactured, prepared, produced, stored, packaged or labelled, on any part of the label other than any part that is applied or attached to the bottom of the container of the food; and
- (c) any other information that is required to be shown on the label of the prepackaged food in accordance with the requirements of the Food and Drug Regulations for any prepackaged product as defined in subsection B.01.001(1) of those Regulations.
Exception — name and principal place of business
(2) The information referred to in paragraph (1)(b) may be shown on any part of the label that is applied or attached to the bottom of the container if that information is also shown on a part of the label that is not applied or attached to the bottom of the container.
Exception — common name
219 (1) The following foods are not required to be labelled with the common name:
- (a) prepackaged fresh fruits or vegetables that are packaged in such a manner that the fresh fruits or vegetables are visible and identifiable in the container; and
- (b) consumer prepackaged fresh apples that are packaged in such a manner that the name of their variety is shown on any part of the label, except if that name is shown on any part that is applied to the bottom of the container.
Definition of apple
(2) In paragraph (1)(b), apple means a fresh apple for which a grade is prescribed by these Regulations.
Exception — name and principal place of business
220 Consumer prepackaged fresh fruits or vegetables that are packaged at retail in such a manner that the fresh fruits or vegetables are visible and identifiable in the container are not required to be labelled with the name and principal place of business referred to in paragraph 218(1)(b).
Consumer Prepackaged Foods
Consumer prepackaged food — declaration of net quantity
221 A label that is applied or attached to a consumer prepackaged food must bear on the principal display panel a declaration of net quantity of the food.
Place of manufacture — label or container
222 If the label that is applied or attached to a consumer prepackaged food bears any reference, direct or indirect, to the place of manufacture of the label or container, the reference to that place must be accompanied by an additional statement that indicates that the reference relates only to the place of manufacture of the label or container.
Name of importer
223 (1) If a consumer prepackaged food was wholly manufactured, processed or produced in a foreign state and the name and principal place of business of the person in Canada for whom it was manufactured, processed or produced or the person by whom it was stored, packaged or labelled in Canada is shown on its label, that information must be preceded by the expressions “Imported by” and “importé par” or “Imported for” and “importé pour”, as the case may be, unless the geographic origin of the consumer prepackaged food is shown on the label in accordance with subsection (3).
Food packaged in Canada
(2) If a food that was wholly manufactured, processed, produced in a foreign state is packaged in Canada, other than at retail, and the name and principal place of business of the person in Canada for whom the food was manufactured, processed, produced or packaged is shown on the label that is applied or attached to the resulting consumer prepackaged food, that information must be preceded by the expressions “Imported by” and “importé par” or “Imported for” and “importé pour”, as the case may be, unless the geographic origin of the food is shown on the label in accordance with subsection (3).
Geographic origin
(3) The geographic origin of a food must, subject to the requirements of any other federal or provincial law, be shown
- (a) in close proximity to the name and principal place of business of the person by or for whom the food was manufactured, processed or produced; and
- (b) in characters of at least the same height as those in which the information referred to in paragraph (a) is shown.
Flavouring ingredient
224 (1) If a flavouring ingredient is added to a consumer prepackaged food, the label that is applied or attached to the consumer prepackaged food must bear a statement that indicates that the flavouring ingredient is imitation, artificial or simulated if
- (a) the ingredient is not derived from a natural substance such as meat, fish, poultry, fruits, vegetables, edible yeast, herbs, spices, bark, buds, roots, leaves or other plant material; and
- (b) the label bears a pictorial representation that suggests the natural food flavour that corresponds to the added flavouring ingredient.
Statement
(2) The statement must be shown
- (a) on or in close proximity to the pictorial representation, if the representation is shown on the principal display panel;
- (b) on the principal display panel, in close proximity to the common name, if the pictorial representation is shown on a part of the label other than the principal display panel; or
- (c) on or in close proximity to the portion of the pictorial representation shown on the principal display panel, if the representation is shown on the principal display panel and on another part of the label.
SUBDIVISION E
Requirement to Apply or Attach Label
Prepackaged food
225 The label of a prepackaged food must be applied or attached in such a manner that the label is still applied or attached at the time it is sold.
Consumer prepackaged food — container
226 Subject to section 228, the label of a consumer prepackaged food that is offered for sale must be applied or attached to the container in accordance with section 227.
Principal display surface
227 (1) All or part of the label of a consumer prepackaged food must be applied to the principal display surface.
Ornamental container
(2) Despite subsection (1), in the case of a consumer prepackaged food whose container is an ornamental container, the label may be applied to the bottom of the container or attached to the container.
Display card
228 In the case of a consumer prepackaged food whose container is mounted on a display card, the label may be applied to the surface of the display card that is displayed or visible under customary conditions of sale or use.
SUBDIVISION F
Type Size — Specific Information
Consumer prepackaged food
229 (1) In the case of the label of a consumer prepackaged food, the following must be shown in characters of at least the minimum character height that is set out in column 2 of Schedule 6 for the area of a principal display surface that is set out in column 1:
- (a) the numerical quantity in the declaration of net quantity; and
- (b) the statement referred to in section 224 that indicates that a flavouring ingredient is imitation, artificial or simulated.
Container mounted on display card — specific case
(2) For the purposes of subsection (1), in the case of a container that is mounted on a display card, the reference to “Area of Principal Display Surface” in Schedule 6 must be read as “Total Area of the Surface of the Display Card that is Displayed or Visible under Customary Conditions of Sale or Use”, if the information is shown on a label that is applied to all or part of that surface.
Consumer prepackaged wine — specific case
(3) Despite paragraph (1)(a), in the case of consumer prepackaged wine, the numerical quantity in the declaration of net quantity may, if the net quantity is 750 mL, the container is no taller than 360 mm and the area of the principal display surface is greater than 258 cm2, be shown in characters of a height less than the minimum character height that is set out in column 2 of Schedule 6, but must be shown in characters that are at least 3.3 mm in height.
SUBDIVISION G
Manner of Showing Declaration of Net Quantity
Legibility
Consumer prepackaged food
230 The declaration of net quantity that is shown on the label of a consumer prepackaged food must
- (a) be in distinct contrast to any other information or pictorial representation on the label; and
- (b) show the numerical quantity in boldface type.
Declaration by Volume, Weight or Numerical Count
General requirements
231 The declaration of net quantity of a consumer prepackaged food must be shown
- (a) in the case of a consumer prepackaged food that is listed in the document entitled Units of Measurement for the Net Quantity Declaration of Certain Foods, prepared by the Agency and published on its website, as amended from time to time, by volume, weight or numerical count in accordance with that document; or
- (b) in the case of a consumer prepackaged food that is not listed in the document referred to in paragraph (a),
- (i) if the food is liquid, gas or viscous, by food volume, or, if the food is solid, by weight, or
- (ii) if the established trade practice in respect of the food is to show its net quantity in a manner that is different than what is required by subparagraph (i), in accordance with that established trade practice.
Metric Units
Permitted units of measurement
232 The declaration of net quantity of a consumer prepackaged food must be shown in metric units, unless otherwise provided by these Regulations.
Millilitres, litres, grams and kilograms
233 (1) The metric units that must be shown in a declaration of net quantity of a consumer prepackaged food must be in
- (a) millilitres, if the net volume of the food is less than 1 000 mL;
- (b) litres, if the net volume of the food is 1 000 mL or more;
- (c) grams, if the net weight of the food is less than 1 000 g; and
- (d) kilograms, if the net weight of the food is 1 000 g or more.
Half-litre or half-kilogram
(2) Despite paragraphs (1)(a) and (c), 500 mL may be shown as 0.5 L and 500 g may be shown as 0.5 kg.
Decimal fraction
(3) In the case referred to in paragraph (1)(c), the net weight may be shown as a decimal fraction of a kilogram if the food is packaged from bulk at retail or is a catch-weight food that is sold by a retailer.
Number of digits
234 (1) If the declaration of net quantity of a consumer prepackaged food is shown in metric units, it must be shown in the decimal system to three figures.
Net quantity below 100 g or mL
(2) Despite subsection (1), if the net quantity is below 100 g or 100 mL, it may be shown to two figures.
Zero as final decimal
(3) Despite subsections (1) and (2), any final zero appearing to the right of the decimal point is not required to be shown.
Quantity less than one
235 If the declaration of net quantity of a consumer prepackaged food is shown in metric units and the quantity is less than one metric unit, the quantity must be shown
- (a) in words; or
- (b) in the decimal system, with a zero preceding the decimal point.
Metric Units and Canadian Units
Grouping
236 If the declaration of net quantity of a consumer prepackaged food is shown in metric units and Canadian units, those units must be grouped together, except that any symbol or pictogram that is shown in accordance with the Canada Consumer Product Safety Act or any regulations made under that Act may be shown between those units.
Canadian units of volume
237 (1) If the declaration of net quantity of a consumer prepackaged food whose volume is less than one gallon includes Canadian units, those units must be in fluid ounces, except that 20 fluid ounces may be shown as one pint, 40 fluid ounces as one quart, 60 fluid ounces as three pints, 80 fluid ounces as two quarts or as one-half gallon and 120 fluid ounces as three quarts.
Oysters
(2) Despite subsection (1), in the case of oysters that are sold in the shell, other than those in a hermetically sealed package, the declaration of net quantity must, if shown by volume, be shown in bushels or pecks.
Net quantity in advertisement
238 If the declaration of net quantity of a consumer prepackaged food or of a serving of the food is shown in metric units and Canadian units, the net quantity of the food or serving in an advertisement may be shown in either a metric unit or a Canadian unit.
Individually Packaged Food Sold as One Unit and Servings
Individually packaged food sold as one unit
239 If a consumer prepackaged food is sold as one unit but consists of two or more individually packaged foods that are labelled with the information required for a consumer prepackaged food, the declaration of net quantity of the consumer prepackaged food being sold as one unit must show
- (a) the number of individually packaged foods in each class of food, as well as the common name of the food in each class; and
- (b) the total net quantity of the individually packaged foods in each class, or the net quantity of each identical individually packaged food in each class.
Prohibition — representation respecting number of servings
240 It is prohibited for a person to apply or attach to any consumer prepackaged food a label that bears any representation with respect to the number of servings contained in the consumer prepackaged food unless the label bears a declaration of net quantity of each serving in accordance with section 241.
Servings
241 (1) The declaration of net quantity of a serving of a consumer prepackaged food must be shown
- (a) in close proximity to the representation with respect to the number of servings contained in the consumer prepackaged food; and
- (b) in characters of the same height as those in which that representation is shown.
Units
(2) The declaration of net quantity of a serving must be shown
- (a) in accordance with the requirements of sections 231 and 233 to 237 respecting the declaration of net quantity of the food; and
- (b) in metric units, unless otherwise provided by these Regulations.
Representation — cups or tablespoons
(3) If the representation with respect to the number of servings is made in terms of cups or tablespoons,
- (a) one cup is equivalent to 250 mL and one tablespoon is equivalent to 15 mL; and
- (b) the declaration of net quantity is not required to meet the requirements of paragraph (2)(b).
DIVISION 3
Specific Requirements for Certain Foods
SUBDIVISION A
Application of Division
Interprovincial trade, import and export
242 The requirements of this Division apply in respect of any food that is sent or conveyed from one province to another, imported or exported.
SUBDIVISION B
Declaration of Net Quantity
Exception — consumer prepackaged food
243 The requirements relating to the declaration of net quantity that are set out in this Division do not apply in respect of a consumer prepackaged food.
Declaration of net quantity
244 Any declaration of net quantity that is required by this Division must be shown by volume, weight or numerical count in accordance with the document entitled Units of Measurement for the Net Quantity Declaration of Certain Foods, prepared by the Agency and published on its website, as amended from time to time.
SUBDIVISION C
Location of Information
Food or container
245 (1) A label that bears the information required by this Division in respect of a food must be applied or attached
- (a) in the case of a prepackaged food, to its container; or
- (b) in the case of food that is not prepackaged, to the food.
Any part of label
(2) The information may be shown on any part of the label, unless otherwise provided by this Division in respect of the food.
Bottom of food or container
(3) Despite subsection (2), if the information is shown on any part of the label that is applied or attached to the bottom of the prepackaged food or container, that information must also be shown
- (a) on any part of the label where it is required to be shown under another provision of this Division in respect of the food; or
- (b) if paragraph (a) does not apply, on any part of the label that is not applied or attached to the bottom of the food or container.
SUBDIVISION D
Dairy Products
Prepackaged dairy products
246 The principal display panel of a prepackaged dairy product must bear
- (a) in the case of butter, calorie-reduced butter, light butter or lite butter, dairy spread and whey butter, the following words or expressions:
- (i) “Cultured” or “de culture”, preceding the common name in English or following the common name in French, if the dairy product has been prepared from cream to which a bacterial culture has been added,
- (ii) “Whipped” or “fouetté”, preceding the common name in English or following the common name in French, if the dairy product has had air or inert gas uniformly incorporated into it as a result of whipping,
- (iii) “Unsalted” or “non salé”, in close proximity to the common name, if the dairy product is unsalted and has not been cultured, and
- (iv) “Salted” or “salé”, in close proximity to the common name, if the dairy product is salted and has been cultured;
- (b) in the case of a combination of skim milk powder and whey powder, the percentage of each powder;
- (c) in the case of partly skimmed milk powder, dairy spread and calorie-reduced butter, the percentage of milk fat; and
- (d) in all cases, a declaration of net quantity that is
- (i) in metric units or Canadian units, or both, in which case the units must be grouped together, if a standard is set out in Volume 1 of the Standards of Identity Document for the dairy product, or
- (ii) in metric units, if no standard is set out in Volume 1 of the Standards of Identity Document for the dairy product.
Prepackaged dairy products — not consumer prepackaged
247 The principal display panel of a prepackaged dairy product, other than a consumer prepackaged dairy product, must bear
- (a) in the case of cheese in its original shape, made from pasteurized milk, the word “Pasteurized” or “pasteurisé”, unless the list of ingredients indicates that the cheese is made from pasteurized milk;
- (b) in the case of buttermilk powder, the percentage of milk fat;
- (c) in the case of skim milk powder that has a whey protein nitrogen content of not less than 6.0 mg/g, the expression “Low Heat” or “Low Temperature” or “basse température” or the abbreviation “Low Temp.” or “basse temp.”; and
- (d) in the case of skim milk powder that has a whey protein nitrogen content of not more than 1.5 mg/g, the expression “High Heat” or “High Temperature” or “haute température” or the abbreviation “High Temp.” or “haute temp.”.
Consumer prepackaged dairy products
248 The principal display panel of a consumer prepackaged dairy product must bear
- (a) in the case of cheese, except cottage cheese and creamed cottage cheese, and in the case of cheese curd, the percentage of moisture;
- (b) in the case of cheese, cheese curd and evaporated partly skimmed milk or concentrated partly skimmed milk, the percentage of milk fat;
- (c) in the case of a dairy product that consists of or was manufactured or prepared wholly or partly from milk that is obtained from an animal other than a cow, the source of the milk, unless the source is indicated in the common name; and
- (d) in the case of a dairy product that is sold as one unit but consists of two or more individual packages of butter patties, butter reddies or other related dairy products, the number of individual packages, as well as the net quantity of each individual package, if the total net quantity of the individual packages is more than 20 g.
Consumer prepackaged cheese
249 (1) The principal display panel of a consumer prepackaged cheese must bear
- (a) the relative firmness of the cheese;
- (b) except in the case of a soft white cheese, the principal ripening characteristic of the cheese;
- (c) in the case of hard cheese that is intended for grating and has a moisture content of 34% or less, the expressions “Hard Grating Cheese” and “fromage dur à râper”; and
- (d) in the case of a mixture of grated or shredded cheeses, the varieties of the cheeses, in descending order of their proportion in the cheese.
Exception
(2) Subsection (1) does not apply in respect of
- (a) cheddar cheese;
- (b) cream cheese with or without added ingredients;
- (c) cream cheese spread with or without added ingredients;
- (d) whey cheese;
- (e) processed cheese with or without added ingredients;
- (f) processed cheese food with or without added ingredients;
- (g) processed cheese spread with or without added ingredients;
- (h) cold-pack cheese with or without added ingredients;
- (i) cold-pack cheese food with or without added ingredients;
- (j) cottage cheese;
- (k) creamed cottage cheese; and
- (l) any cheese that is listed in Part I or II of the table to section B.08.033 of the Food and Drug Regulations.
Relative firmness
(3) The relative firmness of the cheese must be identified by the following expressions:
- (a) “Soft White Cheese” and “fromage à pâte fraîche” or “fromage frais”, if it has a moisture on fat-free basis content of 80% or more;
- (b) “Soft Cheese” and “fromage à pâte molle”, if it has a moisture on fat-free basis content of more than 67% but less than 80%;
- (c) “Semi-soft Cheese” and “fromage à pâte demi-ferme”, if it has a moisture on fat-free basis content of more than 62% but not more than 67%;
- (d) “Firm Cheese” and “fromage à pâte ferme”, if it has a moisture on fat-free basis content of 50% or more but not more than 62%; and
- (e) “Hard Cheese” and “fromage à pâte dure”, if it has a moisture on fat-free basis content of less than 50%.
Principal ripening characteristic
(4) The principal ripening characteristic of the cheese must be identified by the following words or expressions:
- (a) “Ripened” and “affiné”, if the ripening process develops within the whole body of the cheese;
- (b) “Surface Ripened” and “affiné en surface”, if the ripening process starts from the surface and moves into the body of the cheese;
- (c) “Blue Veined” and “à pâte persillée”, if veins of mould occur within the body of the cheese; and
- (d) “Unripened” and “non affiné” or “Fresh” and “frais”, if the cheese has not undergone any ripening.
Imported dairy products
250 (1) The label of the following dairy products must bear the expression “Product of” or “produit de”, followed by the name of the foreign state of origin:
- (a) an imported prepackaged dairy product; and
- (b) a consumer prepackaged cheese that is packaged in Canada from imported bulk cheese for which a standard is set out in Volume 1 of the Standards of Identity Document.
Principal display panel
(2) In the case of the cheese referred to in paragraph (1)(b), the information must be shown on the principal display panel.
Exception
251 Sections 246, 248 and 250 do not apply in respect of an individual portion of a consumer prepackaged dairy product that is sold
- (a) by means of an automatic vending machine or mobile canteen; or
- (b) by a restaurant or other commercial enterprise if it is served with meals or snacks.
Exported prepackaged dairy products
252 The label of a prepackaged dairy product that is exported must bear the expression “Product of Canada” or “produit du Canada”.
Type size
253 The information that is required by sections 250 and 252 must be shown in boldface type in characters that are at least 16 mm in height, in the case of a prepackaged dairy product other than a consumer prepackaged dairy product.
SUBDIVISION E
Eggs
Graded eggs
254 The label of prepackaged eggs that are graded in accordance with these Regulations must bear
- (a) a declaration of net quantity; and
- (b) in the case of eggs that are pasteurized in the shell, the words “Pasteurized” and “pasteurisé”, as well as the expressions “Graded Canada A Before Pasteurization” and “classifié Canada A avant pasteurisation” or the expressions “Graded Grade A Before Pasteurization” and “classifié catégorie A avant pasteurisation”, as the case may be.
Size of label of graded egg
255 The label applied to an egg that is graded Canada A, Canada B, Grade A or Grade B must not cover an area of the egg that is larger than 2.5 cm2.
Imported eggs
256 (1) The label of imported prepackaged eggs must bear the expressions “Product of” and “produit de”, followed by the name of the foreign state of origin.
Location and type size
(2) That information must be shown in characters that are
- (a) in the case of a tray with an overwrap or an egg carton, on the top or side of the tray or egg carton, at least 1.5 mm in height; and
- (b) in the case of a container other than a tray with an overwrap or an egg carton, at least 6 mm in height.
Eggs to be exported
257 (1) The label of prepackaged eggs that are graded in accordance with these Regulations and that are exported must bear the expressions “Product of Canada” and “produit du Canada”.
Location and type size
(2) That information must be shown in characters that are
- (a) in the case of a tray with an overwrap or an egg carton, on the top or side of the tray or egg carton, at least 1.5 mm in height; and
- (b) in the case of a container other than a tray with an overwrap or an egg carton, immediately below the common name, at least 13 mm in height.
SUBDIVISION F
Processed Egg Products
Prepackaged processed egg products
258 The label of a prepackaged processed egg product must bear
- (a) the inspection legend set out in Figure 1 of Schedule 2, if the prepackaged processed egg product is sent or conveyed from one province to another or exported;
- (b) the official inspection mark of the foreign state of origin, if the prepackaged processed egg product is imported;
- (c) the expression “Product of Turkey Eggs” or “produit d’œufs de dinde” or the expression “Product of Turkey Eggs and Chicken Eggs” or “produit d’œufs de dinde et de poule”, as the case may be, if the processed egg product was manufactured or prepared from eggs of a domestic turkey or from eggs of a domestic turkey and eggs of a domestic chicken; and
- (d) the expression “Pan-dried” or “séché sur plaque” or the expression “Spray-dried” or “séché par pulvérisation”, as the case may be, if the processed egg product is dried egg white or dried albumen.
Imported prepackaged processed egg products
259 The label of an imported prepackaged processed egg product must also bear the expression “Product of” or “produit de”, followed by the name of the foreign state of origin.
Prepackaged dried egg blends
260 The label of the following prepackaged processed egg products must bear the expression “Product of Canada and” or “produit du Canada et”, followed by the name of the foreign state of origin:
- (a) dried whole egg that is a blend of imported and Canadian dried whole egg;
- (b) dried yolk that is a blend of imported and Canadian dried yolk; and
- (c) dried egg white or dried albumen that is a blend of imported and Canadian dried egg white or dried albumen.
SUBDIVISION G
Fish
Definitions
261 The following definitions apply in this Subdivision.
brine means sea water, with or without the addition of salt, or a solution of salt and fresh water. (saumure)
fillet means a slice of fish flesh of irregular size and shape, whether cut into sections or not, that
- (a) has been removed from the carcass of a fish by cuts that are parallel to the backbone; and
- (b) has had the internal organs, head, fins and all discoloured flesh and bones, other than intramuscular or lateral bones, removed. (filet)
minced, in respect of fish, means that particles of skeletal muscle have been separated from a clean, sound fish that has had its head and all its internal organs, bones, skin and discoloured flesh removed. (haché)
salted fish means fish of the Gadidae family that has been preserved by salt and that has a salt content of 12% or more by wet weight and a moisture content of not more than 65%. (poisson salé)
whitefish means fish of the species Coregonus clupeaformis, Coregonus nasus or Prosopium cylindraceum. (poisson blanc)
Prepackaged fish
262 (1) The label of prepackaged fish must bear
- (a) in the case of salmon that is in a hermetically sealed package, the word “Skinless” or the expression “sans peau” or the word “Boneless” or the expression “sans os” or “sans arête”, if the skin and the vertebrae have been removed from the salmon and the salmon consists of sections of flesh that are cut transversely from the fish and are nearly equal in length to the height of the hermetically sealed package;
- (b) in the case of minced salmon or trimmings from the tail and nape sections of a salmon or other small pieces of salmon, the word “Minced” or the expression “haché” or the expression “Salmon Tips” or “bouts de saumon”, as the case may be, if the salmon or trimmings are in a hermetically sealed package;
- (c) in the case of unfrozen lobster meat that has been packaged without the addition of brine, the expression “Dry Pack” or “emballage à sec”;
- (d) in the case of frozen lobster meat, the expression “Frozen Lobster Meat” or “chair de homard congelée”;
- (e) in the case of fish sticks, fish fingers and other uniform rectangular portions of breaded fish flesh that were manufactured or prepared from minced fish, a descriptive term declaring that the food is manufactured or prepared from minced fish;
- (f) in the case of bivalve molluscs in the shell that are not in a hermetically sealed package, the date of processing and an expression, code or identifier that indicates the area from which the bivalve molluscs were harvested;
- (g) in the case of tuna that is in a hermetically sealed package, one of the following expressions to describe the colour of the fish flesh:
- (i) “White Meat Tuna” or “chair de thon blanc” or “White Tuna” or “thon blanc”, if the tuna is of the species Thunnus alalunga and has a diffuse luminous reflectance of not less than 33.7% of that of magnesium oxide,
- (ii) “Light Meat Tuna” or “chair pâle de thon” or “Light Tuna” or “thon pâle”, if the tuna has a diffuse luminous reflectance of not less than 22.6% of that of magnesium oxide, and
- (iii) “Dark Meat Tuna” or “chair foncée de thon” or “Dark Tuna” or “thon foncé”, if the tuna does not meet the requirements of subparagraph (ii);
- (h) in the case of salted fish, one of the following words or expressions to describe the processing of the fish:
- (i) “Split Fish” or “poisson fendu”, if the fish is split and at least two thirds of the anterior of the backbone has been removed,
- (ii) “Split Fish with Entire Backbone” or “poisson fendu avec colonne vertébrale entière”, if the fish is split and no portion of the backbone has been removed,
- (iii) “Fillet” or “filet”, in the case of a fillet as defined in section 261, and
- (iv) any other expression that is distinctive from those set out in subparagraphs (i) to (iii) and that describes the processing of the fish;
- (i) in the case of salted fish, one of the following expressions to describe the salt or moisture content of the fish:
- (i) “Slack Salted Fish” or “poisson faiblement salé”, if, after salting is complete, the fish has a salt content of not more than 25% by dry weight,
- (ii) “Light Salted Fish” or “poisson légèrement salé”, if, after salting is complete, the fish has a salt content of more than 25% but not more than 33% by dry weight,
- (iii) “Dried Heavy Salted Fish” or “poisson fortement salé séché”, if, after salting is complete, the fish has a salt content of more than 33% by dry weight and has a moisture content of not more than 54%, and
- (iv) “Green Heavy Salted Fish” or “poisson fortement salé en vert”, if, after salting is complete, the fish has a salt content of more than 33% by dry weight and has a moisture content of more than 54% but not more than 65%;
- (j) in the case of fish that is in a hermetically sealed package, an indication, as part of the common name, as to whether the fish was manufactured or prepared
- (i) by mincing, flaking or another special process,
- (ii) from selected parts of fish, or
- (iii) for dietetic use; and
- (k) in all cases, a declaration of net quantity.
Mackerel
(2) In the case of mackerel or mackerel fillets that are packaged without the addition of water, brine or a vinegar solution and that are in a hermetically sealed package, the label must bear the drained weight in addition to the declaration of net quantity, if the drained weight is less than 80% of that quantity.
Descriptive terms — minced fish
(3) The descriptive term referred to in paragraph (1)(e) must be shown in close proximity to the common name and in characters that are at least the height that is the greater of
- (a) one-half the height of the characters in which the common name is shown, and
- (b) 1.6 mm.
Prepackaged fish placed in a second container
263 If prepackaged fish that is labelled in accordance with this Part is placed inside of a second container and the resulting product is prepackaged fish, other than consumer prepackaged fish, the second container is not required to be labelled with the declaration of net quantity referred to in paragraph 262(1)(k).
Prepackaged fish — common name
264 If prepackaged fish is of a species that is set out in the document entitled Common Names for Prepackaged Fish, prepared by the Agency and published on its website, as amended from time to time, the common name that is required to be shown on the label is any common name that is set out for that species in that document.
Fish in hermetically sealed package
265 In the case of fish that is in a hermetically sealed package and commercially sterile, the declaration of net quantity referred to in paragraph 262(1)(k) must be shown on the principal display panel.
Imported prepackaged fish
266 The label of imported prepackaged fish must bear the name of the foreign state of origin.
Prepackaged whitefish
267 The label of prepackaged whitefish, other than imported prepackaged whitefish, must bear the name of the lake and province of origin.
SUBDIVISION H
Fresh Fruits or Vegetables
Prepackaged fresh fruits or vegetables
268 (1) The label of prepackaged fresh fruits or vegetables must bear
- (a) in the case of apples, the name of the variety; and
- (b) in all cases, a declaration of net quantity.
Prepackaged apples placed in second container
(2) If prepackaged fresh apples that are labelled in accordance with this Part are placed inside of a second container and the resulting product is prepackaged fresh apples, other than consumer prepackaged fresh apples, the second container is not required to be labelled with the name of the variety.
Definition of apple
(3) In paragraph (1)(a) and subsection (2), apple means a fresh apple for which a grade is prescribed by these Regulations.
Declaration of net quantity
(4) Unless the declaration of net quantity is shown by numerical count, it must be shown in metric units or Canadian units, or both, in which case the units must be grouped together.
Imported prepackaged fresh fruits or vegetables
269 (1) The expression “Product of” or “Produce of” or “produit de”, “Grown in” or “cultivé dans” or “Country of Origin” or “pays d’origine”, followed by the name of the foreign state in which the fresh fruits or vegetables were grown, or other words that clearly indicate that foreign state, must be shown on the principal display panel of imported prepackaged fresh fruits or vegetables in close proximity to the declaration of net quantity or the grade name.
Prepackaged fresh fruits or vegetables placed in second container
(2) If prepackaged fresh fruits or vegetables that are labelled in accordance with this Part are placed inside of a second container and the resulting product is prepackaged fresh fruits or vegetables, other than consumer prepackaged fresh fruits or vegetables, the second container is not required to be labelled with the information referred to in subsection (1) if that information is readily discernible and legible without having to open the second container and that information is not obscured by the container.
Subsequent repackaging
(3) This section applies whether or not the imported prepackaged fresh fruits or vegetables are subsequently repackaged in Canada.
Type size
270 (1) The information that is required by section 269 must be shown in boldface type in characters of at least the minimum character height that is set out in column 2 of Schedule 6 for the area of a principal display surface that is set out in column 1.
Exception
(2) Subsection (1) does not apply in respect of consumer prepackaged fresh fruits or vegetables that are packaged from bulk at retail or that are catch-weight foods sold by a retailer.
Reusable plastic container
271 Despite subsection 270(1), in the case of prepackaged fresh fruits or vegetables, other than consumer prepackaged fresh fruits or vegetables, whose container is a reusable plastic container, the characters must be at least 1.6 mm in height.
SUBDIVISION I
Processed Fruit or Vegetable Products
Prepackaged processed fruit or vegetable products
272 (1) The label of a prepackaged processed fruit or vegetable product must bear
- (a) a declaration of net quantity, in metric units, shown on the principal display panel;
- (b) the expression “Solid Pack” or “conserve compacte”, in the case of a package containing fruits or vegetables that have been partially or wholly precooked before processing so as to allow the fruits or vegetables to pack closely with the minimum amount of free liquid and in which there is little or no free liquid;
- (c) the expression “Heavy Pack” or “conserve épaisse”, in the case of a package in which the minimum amount of water required for proper processing of the product is used as the liquid packaging medium and that contains the maximum drained weight of the food that processing will permit;
- (d) the expression “In Water” or “dans l’eau”, if the product is packaged in water;
- (e) the expression “Contents ... Per Cent Slack Filled” or “... pour cent du contenant non rempli” or “Contents ... Per Cent Short Weight” or “contient ... pour cent de moins que le poids indiqué”, if the package is not filled to capacity or contains less than the minimum net and drained weights prescribed by these Regulations;
- (f) the total percentage of sweetening ingredients added, if any, in the case of frozen fruits packaged in sugar, invert sugar, dextrose or glucose in dry form;
- (g) the word “Seville” or “Séville” or “Bitter” or “amère” or the expression “Extra Bitter” or “extra amère”, in the case of orange marmalade made from Seville or other bitter varieties of oranges;
- (h) in the case of green or wax beans, whether they are frozen or in a hermetically sealed package, any of the following to describe the style of cut or packaging:
- (i) the word “Whole” or “entiers”, in the case of whole beans that are not arranged in any definite position in a package,
- (ii) the expression “Asparagus Style” or “genre asperges” or “Whole Vertical Pack” or “entiers, emballage vertical”, in the case of whole beans that are packaged parallel to the sides of a package and are substantially equal in length,
- (iii) the word “Cut” or “coupés”, in the case of pods that are cut transversely into pieces that are not more than 51 mm in length and not less than 19 mm in length, except in the case of shorter end pieces that result from cutting, and
- (iv) the expression “French Cut” or “coupe française” or “French Style” or “à la française”, in the case of pods that are sliced lengthwise;
- (i) the expression “Tips Removed” or “pointes enlevées” or “Without Tips” or “sans pointes” immediately below the common name, in the case of asparagus cuts or cuttings that are graded Canada Choice and packaged without tips;
- (j) in the case of corn that is in a hermetically sealed package, any of the following:
- (i) the expression “Cream Style” or “maïs crème” or “Packed in Liquid” or “conservé dans un liquide”, as the case may be,
- (ii) the expression “Brine Pack” or “mis en conserve dans la saumure” or “Packed in Brine” or “conservé dans la saumure” or “in Brine” or “dans la saumure”, if the corn is in a package in which a water and salt solution is used as the liquid packaging medium with or without the addition of sugar, and
- (iii) the expression “Vacuum Pack” or “mis en conserve sous vide” or “Vacuum Packed” or “conservé sous vide”, if the corn is in a package in which a minimum amount of liquid packaging medium is used and in which a vacuum is created mechanically;
- (k) the expression “Vitamin C Added” or “additionné de vitamine C” or the word “Vitaminized” or “vitaminé”, in the case of apple juice, mixed vegetable juice, tomato juice cocktail, prune nectar, apricot nectar, grape juice or grape juice from concentrate, to which ascorbic acid has been added in order to increase the Vitamin C content;
- (l) the expression “A Water Extract of Dried Prunes” or “extrait aqueux de pruneaux secs” immediately following the common name, in the case of prune nectar;
- (m) the word “Clingstone” or the expression “à noyau adhérent”, in the case of peaches that are in a hermetically sealed package and that have stones or pits that adhere to the flesh, or the word “Freestone” or the expression “à noyau non adhérent”, in the case of peaches that are in a hermetically sealed package and whose flesh separates readily from the stones or pits;
- (n) the expression “Keep Refrigerated” or “garder réfrigéré”, in the case of sauerkraut with preservative, or a fruit juice that is in a non-hermetically sealed package;
- (o) the word “Wild” or the expression “de type sauvage” or the word “Cultivated” or the expression “de type cultivé”, as the case may be, as well as the abbreviation “I.Q.F.” (Individually Quick Frozen) or the word “surgelés” or the expression “Non-free Flowing” or “non individuellement congelés”, in the case of frozen blueberries;
- (p) the word “Sparkling” or “pétillant”, “mousseux” or “gazéifié” or “Carbonated” or “carbonaté”, “mousseux” or “gazéifié”, in the case of apple juice, apple juice from concentrate, grape juice or grape juice from concentrate, to which carbon dioxide under pressure has been added; and
- (q) the word “Pitted” or “dénoyautées”, in the case of frozen sweet cherries that are whole and stemmed and that have had the pits removed, or the word “Unpitted” or the expression “non dénoyautées”, in the case of frozen sweet cherries that are whole and stemmed and that have not had the pits removed.
Definition of sweetening ingredient
(2) In paragraph (1)(f), sweetening ingredient means white sugar, brown sugar, yellow sugar, golden sugar, liquid sugar, invert sugar, honey, cane sugar, maple sugar, maple syrup, molasses, refined sugar syrup, refiner’s syrup, golden syrup, corn syrup, glucose, dextrose, fructose or any combination of those substances in dry or liquid form.
Identification name
273 A food that is set out in column 1 of Schedule 7 that is frozen or in a hermetically sealed package, that is packaged in syrup or fruit juice, or in fruit juice to which sugar has been added, and that has a percentage of soluble solids that is set out in any of paragraphs (a) to (e) of column 2 must be labelled with the identification name that is set out for that percentage in column 3.
Name of foreign state
274 (1) The label of an imported prepackaged processed fruit or vegetable product must bear the name of the foreign state where the processed fruit or vegetable product was packaged.
Type size
(2) The name must be shown in characters that are at least 1.6 mm in height.
Product packaged for Canadian importer
(3) Despite subsection (2), if the product was packaged for a Canadian importer under the importer’s private label, the name must be shown in characters that are
- (a) at least 6.4 mm in height, if the declaration of net quantity is more than 283.5 g; and
- (b) at least 3.2 mm in height, if the declaration of net quantity is 283.5 g or less.
SUBDIVISION J
Honey
Prepackaged honey
275 (1) The label of prepackaged honey that is graded must bear
- (a) a declaration of net quantity, in metric units or, in the case of prepackaged honey that is sold as one unit but that consists of two or more individual packages, the number of those packages and the net quantity of each, in metric units; and
- (b) the word “Creamed” or “en crème” or another word that indicates that the contents are granulated, “Liquid” or “liquide”, “Pasteurized” or “pasteurisé” or “Pressed” or “de presse”, as the case may be.
Location
(2) In the case of consumer prepackaged honey, the information referred to in paragraph (1)(b) must be shown on the principal display panel.
Graded Canadian honey
276 The label of prepackaged honey that is produced in Canada and graded in accordance with these Regulations must bear the expression “Product of Canada” or “produit du Canada” or “Canadian Honey” or “miel canadien”.
Imported prepackaged honey
277 (1) The label of imported prepackaged honey must bear the expression “Product of” or “produit de” followed by the name of the foreign state of origin.
Type size
(2) In the case of imported prepackaged honey, other than consumer prepackaged honey, that information must be shown in characters that are at least 9.5 mm in height.
Honey packaged from imported honey
278 The label of consumer prepackaged honey that was packaged from imported honey and graded in accordance with these Regulations must bear the expressions “Product of” and “produit de” followed by the name of the foreign state of origin.
Blend of Canadian and imported honey
279 (1) The label of prepackaged honey that is a blend of imported honey and Canadian honey and that is graded in accordance with these Regulations must bear the expression “A Blend of Canadian and (naming the foreign state or states of origin) Honey” or “mélange de miel canadien et de miel (indication de l’État étranger ou des États étrangers d’origine)” or “A Blend of (naming the foreign state or states of origin) Honey and Canadian Honey” or “mélange de miel (indication de l’État étranger ou des États étrangers d’origine) et de miel canadien”.
Sources of honey
(2) The states of origin, Canadian or foreign, must be shown in descending order of the proportion of honey from each state.
SUBDIVISION K
Maple Products
Net quantity
280 (1) The label of a prepackaged maple product must bear a declaration of net quantity in metric units.
Exception
(2) Subsection (1) does not apply in respect of maple syrup unless it is graded in accordance with these Regulations.
Imported maple products
281 The label of the following maple products must bear the name of the foreign state of origin:
- (a) any imported prepackaged maple syrup whose net quantity is 5 L or less; and
- (b) any other imported prepackaged maple product whose net quantity is 5 kg or less.
SUBDIVISION L
Edible Meat Products
Inspection legend — non-prepackaged edible meat products
282 (1) An edible meat product that is not prepackaged must bear
- (a) the inspection legend set out in Figure 1 of Schedule 2, if the edible meat product is sent or conveyed from one province to another or exported; and
- (b) the official inspection mark of the foreign state of origin, if the edible meat product is imported.
Size of inspection legend
(2) If an inspection legend or official inspection mark of a foreign state is applied directly on an edible meat product, the transverse axis passing through the centre of the legend or mark must be at least 25 mm in length.
Label — non-prepackaged edible meat products
283 (1) A label must be applied or attached to an edible meat product that is not prepackaged, and must bear
- (a) the information required by paragraphs 218(1)(a) and (b) and 286(a) and (b) and the expressions mentioned in paragraphs 286(c) and (d), shown on the principal display panel;
- (b) the ingredients listed either in descending order of their proportion in the product or as a percentage of the product; and
- (c) the components of the ingredients,
- (i) listed immediately after the ingredient of which they are components, in such a manner as to indicate that they are components of that ingredient,
- (ii) listed either in descending order of their proportion in the ingredient or as a percentage of the ingredient, and the order or percentage must be the order or percentage of the components before they are combined to form the ingredient, and
- (iii) shown in accordance with sections B.01.009 and B.01.010 of the Food and Drug Regulations.
Exception
(2) If one or more components of an ingredient is shown in a list of ingredients, the ingredient is not required to be shown in the list if all of the components of the ingredient are shown in the list by their common names and in accordance with paragraph (1)(b) as if they were ingredients.
Exception — omission or substitution of an ingredient or ingredient component
284 (1) In the case where it is an acceptable manufacturing practice for a licence holder to omit, from an edible meat product, any food that is ordinarily an ingredient of the meat product or a component of the ingredient, or substitute in whole or in part, in a meat product, any food for a food that is ordinarily an ingredient or a component of the ingredient, the list of ingredients on the label of the meat product may, for the 12-month period beginning at the time the label is applied or attached to the edible meat product, show as ingredients of the meat product or components of the ingredients the food that may be omitted or substituted, if
- (a) all the foods that may be used as ingredients or components throughout the 12-month period are shown in the list of ingredients;
- (b) it is clearly stated as part of the list of ingredients that the food shown as an ingredient or component may not be present in the meat product or that another food may be substituted; and
- (c) the food that is omitted or substituted is grouped with foods of the same class of foods that are used as ingredients or components in the product and the foods within each of the groups of foods are listed in descending order of the proportion in which they are likely to be used during the 12-month period.
Exception — varying proportions
(2) In the case where it is an acceptable manufacturing practice for a licence holder to vary the proportions of ingredients of an edible meat product or components of the ingredients, the list of ingredients on the label of the edible meat product may, for the 12-month period beginning at the time the label is applied or attached to the meat product, show the ingredients or components in the same proportions throughout the 12-month period, if
- (a) it is clearly stated as part of the list of ingredients that the proportions indicated are subject to change; and
- (b) the ingredients or components are listed in descending order of the proportion in which they are likely to be used during the 12-month period.
Definitions
285 The following definitions apply in sections 283 and 284.
component means an individual unit of food that is combined as an individual unit of food with one or more other individual units of food to form an ingredient. (constituant)
ingredient means an individual unit of food that is combined as an individual unit of food with one or more other individual units of food to form an integral unit of food that is an edible meat product. (ingrédient)
Prepackaged edible meat products
286 The label of a prepackaged edible meat product must bear on the principal display panel
- (a) a declaration of net quantity, in metric units, shown in the manner required by sections 233 to 236 and 239 as if it were a consumer prepackaged food;
- (b) a statement that indicates that the meat product must be kept refrigerated or kept frozen, as the case may be, unless the meat product
- (i) is in a hermetically sealed package and is commercially sterile,
- (ii) is dried to attain a water activity of 0.85 or less,
- (iii) has a pH of 4.6 or less,
- (iv) is packaged in salt or a saturated salt solution, or
- (v) is fermented and has a pH of 5.3 or less, and a water activity of 0.90 or less, at the end of the fermentation;
- (c) in the case of a poultry carcass that is dressed or partially dressed, the expressions “With Giblets” and “avec abats” or “avec abattis”, if giblets are packaged with the poultry carcass and it has been graded; and
- (d) in the case of a carcass of a chicken or young duck that is dressed or partially dressed or a portion of such a carcass, the expression “May Contain Kidneys” or “peut contenir les reins”, if the kidneys have not been removed or it may contain kidneys.
Inspection legend — prepackaged edible meat products
287 (1) The label of a prepackaged edible meat product must also bear
- (a) the inspection legend set out in Figure 1 or 2 of Schedule 2, if the prepackaged edible meat product is sent or conveyed from one province to another or exported; and
- (b) the official inspection mark of the foreign state of origin, if the prepackaged edible meat product is imported.
Principal display panel
(2) In the case of a prepackaged edible meat product, other than a consumer prepackaged meat product, the principal display panel must bear the inspection legend or the official inspection mark of the foreign state of origin.
Tamper-resistant seal
(3) Despite subsection (2), in the case of a prepackaged edible meat product, other than a consumer prepackaged meat product, the inspection legend or the official inspection mark of the foreign state of origin may be shown on the tamper-resistant seal, if such a seal is used, unless that seal is applied to the bottom of the container.
Edible meat products
288 (1) The label of an edible meat product may bear a word or expression that is set out in quotation marks in column 1 of Schedule 8 only if the edible meat product meets the requirements that are set out in column 2.
Location
(2) If such a word or expression is shown on the label, it must be shown in close proximity to the common name.
Animal species
289 The label of an edible meat product may describe the meat product as, or as being derived from, a carcass or part of a carcass or a cut, organ or tissue of a food animal only if the label bears the name of the animal species, as it is commonly known, from which the meat product was derived.
Ready-to-eat edible meat products
290 The label of an edible meat product may bear a word or expression that indicates or suggests that it is a ready-to-eat product only if the requirements of section 47 are met in respect of the edible meat product.
Uncooked meat products
291 The principal display panel of a prepackaged edible meat product that is not a ready-to-eat product but could be mistaken for one must bear
- (a) the expression “Must Be Cooked” or “doit être cuit” or “Raw Product” or “produit cru” or the word “Uncooked” or “non cuit”, or any equivalent word or expression, in close proximity to the common name, to indicate that the meat product requires cooking before consumption; and
- (b) comprehensive cooking instructions — such as a combination of internal temperature and cooking time — that, if followed, will result in a ready-to-eat meat product.
Prepackaged poultry carcass
292 In the case of a prepackaged poultry carcass, if the carcass is dressed or partially dressed and has been graded, the common name must be shown on
- (a) the part of the package that lies on or over the anterior centre of the breast of the poultry, if the carcass is individually packaged; or
- (b) on a tag that is attached to the V of the wishbone of the poultry carcass, if it is not individually packaged.
Consumer prepackaged poultry carcass
293 In the case of a consumer prepackaged poultry carcass, if the carcass is dressed or partially dressed and has been graded in accordance with these Regulations, the label must bear
- (a) if the poultry carcass has been basted, the words “Basted” and “imprégné”, the words “Pre-basted” and “préimprégné”, the expressions “Deep Basted” and “imprégné en profondeur” or the words “Self-basting” and “auto-imprégné”, as the case may be, as well as the expressions “Graded before Basting” and “classifié avant imprégnation”;
- (b) if it is graded Canada Utility, the expressions “May Have Parts Missing” and “des parties peuvent manquer”;
- (c) if its breast bone has been removed, the expressions “Breast Bone Removed” and “bréchet enlevé”;
- (d) if it has been stuffed, the words “Stuffed” and “farci” and the expressions “Graded before Stuffing” and “classifié avant d’être farci”; and
- (e) if it has been seasoned, the words “Seasoned” and “assaisonné” and the expressions “Graded before Seasoning” and “classifié avant assaisonnement”.
Poultry carcass — not individually packaged
294 In the case of a prepackaged poultry carcass, if the carcass is dressed or partially dressed and has been graded, but it is not individually packaged, a tag must be attached to the V of the wishbone of the poultry carcass that bears
- (a) the name and principal place of business of the person by or for whom the poultry carcass was packaged; and
- (b) the expression “May Contain Kidneys” or “peut contenir les reins”, if the poultry carcass is that of a chicken or young duck, or a portion of such a carcass, and it may contain kidneys or the kidneys have not been removed.
Word “ham”
295 The label of an edible meat product may bear the word “Ham” or “jambon” only if the product is derived from the hind leg of a dressed swine carcass above the tarsal joint.
Label of edible meat products — exception
296 (1) An edible meat product whose label does not meet the requirements of these Regulations may be sent or conveyed from an establishment that is identified in a licence if
- (a) it is a prepackaged meat product, other than a consumer prepackaged meat product, that is sealed with a tamper-resistant seal or it is in a conveyance that is sealed with a tamper-resistant seal;
- (b) it is sent or conveyed to another establishment where meat products are manufactured, processed, treated, preserved, graded, packaged or labelled by a licence holder; and
- (c) it is accompanied by
- (i) a document from the licence holder that states that the meat product is identified as edible under section 125, and
- (ii) a list of ingredients that meets the requirements of the Food and Drug Regulations that apply in respect of a prepackaged product as defined in subsection B.01.001(1) of those Regulations.
Tamper-resistant seal
(2) The tamper-resistant seal on the prepackaged meat product or conveyance must not be broken until after the meat product arrives at the other establishment.
Imported edible meat products
297 (1) The label of an imported edible meat product must bear the expression “Product of” or “produit de”, followed by the name of the foreign state of origin, in close proximity to the common name.
Type size
(2) The information must, whether or not the edible meat product is a consumer prepackaged meat product, be shown in characters of the height required by subsections 210(2) and (3).
Subsequent packaging or labelling
(3) This section applies whether or not the imported edible meat product is subsequently packaged or labelled in Canada without being manufactured or prepared in Canada.
Imported consumer prepackaged poultry carcasses
298 In the case of an imported consumer prepackaged poultry carcass, if the carcass is dressed or partially dressed and has been graded in accordance with these Regulations, the information required by subsection 297(1) must be shown in the same colour as the grade name.
DIVISION 4
Exceptions
Consumer prepackaged food
299 Sections 199, 200, 216, 221 to 224 and 228 to 241 do not apply in respect of a consumer prepackaged food that is
- (a) manufactured, prepared, produced, packaged or labelled for use by commercial or industrial enterprises or institutions without being sold by them as a consumer prepackaged food;
- (b) manufactured, prepared, produced, packaged or labelled only for sale to or by a duty free shop; or
- (c) distributed to one or more persons for no consideration.
Declaration of net quantity
300 The following consumer prepackaged foods are not required to be labelled with the declaration of net quantity referred to in section 221:
- (a) an individual portion of a food that is prepared by a commissary and sold by automatic vending machine or mobile canteen;
- (b) a catch-weight food that is sold to a retailer; and
- (c) an individual portion of a food that is sold by a restaurant or other commercial enterprise if it is served with meals or snacks.
Raspberries or strawberries
301 Sections 216, 221 and 240 do not apply in respect of consumer prepackaged raspberries or consumer prepackaged strawberries that are packaged in the field in a container that has a capacity of 1.14 L or less.
Individually measured food
302 (1) A declaration of net quantity of a consumer prepackaged food that is an individually measured food is not required to meet the legibility and character height requirements of subsection 210(2), paragraph 229(1)(a), subsections 229(2) and (3) and paragraph 230(b).
Food packaged from bulk
(2) The declaration of net quantity of a consumer prepackaged food, other than an individually measured food, that is packaged from bulk at retail and that is clearly shown on the principal display panel in Canadian units, is not required to
- (a) meet the legibility and character height requirements of subsection 210(2), paragraph 229(1)(a), subsections 229(2) and (3) and paragraph 230(b); or
- (b) be shown in metric units.
Definition of individually measured
(3) In this section, individually measured, in respect of a food, means that the food is measured and packaged in a manner other than in accordance with a predetermined fixed quantity and, as a result, is sold in varying quantities.
Individually packaged food sold as one unit
303 A label of a consumer prepackaged food is not required to meet the requirements of sections 221, 239 and 240 if
- (a) the consumer prepackaged food is sold as one unit but consists of fewer than seven identical individually packaged foods;
- (b) each of those individually packaged foods is labelled with the information required by this Part; and
- (c) that information is readily discernable and legible at the time of sale.
PART 12
Grades and Grade Names
DIVISION 1
Interpretation
Definitions
304 The following definitions apply in this Part.
beef carcass has the same meaning as in the Grades Document. (carcasse de bœuf)
bison carcass has the same meaning as in the Grades Document. (carcasse de bison)
grader means a person designated as a grader under subsection 13(3) of the Canadian Food Inspection Agency Act for the purposes of the Act. (classificateur)
grade roller means a tool that is used to apply a roller brand on each side of a livestock carcass. (rouleau à estampiller)
Grades Document means the document entitled Beef, Bison and Veal Carcass Grade Requirements, prepared by the Canadian Beef Grading Agency and published on its website, as amended from time to time. (Document de classification)
grade stamp means a mark that is applied to a livestock carcass and that shows the grade name and the grader’s code. (cachet de classification)
grade stamp applicator means a tool that is used to apply a grade stamp or a yield stamp to a livestock carcass. (applicateur de cachet de classification)
grading stand means a platform that is used for grading livestock carcasses. (plate-forme de classification)
identification code means a distinct code that is applied to a food animal before slaughter and grading to enable the food animal to be traced. (code d’identification)
knife-rib means to cut the left side, or the left and right sides, of a beef carcass or bison carcass in the following locations by severing the vertebrae and cutting 15 cm or more beyond the longissimus muscles in order to expose those muscles for evaluation by a grader:
- (a) in the case of a beef carcass, between the 12th and 13th ribs; and
- (b) in the case of a bison carcass, between the 11th and 12th ribs. (incision transversale)
lot means a group of food animals or a quantity of livestock carcasses that, for any reason, is considered together for inspection. (lot)
marketing agency means a board or commission that is established under an Act of a province that regulates the marketing of bovine or ovine animals. (office de commercialisation)
meat inspection stamp means
- (a) an inspection legend that is prescribed under section 179 in respect of a meat product; or
- (b) a mark that is authorized under an Act of a province to be applied to or used in connection with a livestock carcass or poultry carcass after inspection. (cachet d’inspection de viande)
musculature means the size and shape of the muscles of a livestock carcass. (musculature)
ovine carcass has the same meaning as in the Compendium. (carcasse d’ovin)
primal cut means
- (a) in the case of a beef carcass or bison carcass, the round, sirloin, short loin, rib or chuck of the carcass side; and
- (b) in the case of an ovine carcass or veal carcass, the leg, loin or foresaddle of the carcass side. (coupe primaire)
producer means any person who sells livestock for slaughter. (producteur)
provincial establishment means an establishment
- (a) that is registered under an Act of a province that regulates the inspection of livestock carcasses or poultry carcasses; or
- (b) where livestock carcasses or poultry carcasses are prepared by any person who is authorized to do so under an Act of a province that regulates the inspection of those carcasses. (établissement provincial)
roller brand means the mark that is applied to a beef carcass and that shows the grade name and the number that is assigned to the establishment where the livestock carcass is graded. (marque d’estampillage)
sub-primal cut means a cut of meat that is greater than 125 cm3 and that is derived from a beef carcass or a primal cut of a beef carcass. (coupe sous-primaire)
trim means to remove all or part of the external fat from a livestock carcass. (parer)
veal carcass has the same meaning as in the Grades Document. (carcasse de veau)
weighmaster means an employee of an establishment that is identified in a licence or of a provincial establishment who is trained to operate a scale that is approved under section 3 of the Weights and Measures Act. (peseur)
yield class has the same meaning as in the Compendium or the Grades Document. (catégorie de rendement)
yield stamp has the same meaning as in the Compendium or the Grades Document. (cachet de rendement)
DIVISION 2
Grade Names
Definition grade name in Act
305 For the purposes of the definition grade name in section 2 of the Act, the grade names that are set out in the Compendium and in the Grades Document are prescribed in respect of foods.
DIVISION 3
Grading
Mandatory grading
306 (1) Any eggs, fish, fresh fruits or vegetables, processed fruit or vegetable products, honey, maple syrup or beef carcass in respect of which grades are prescribed by these Regulations that are sent or conveyed from one province to another or that are imported or exported must
- (a) be graded;
- (b) meet the requirements that are set out in the Compendium or the Grades Document in respect of the applicable grade of that food; and
- (c) be labelled, in accordance with the Compendium or the Grades Document, with the applicable grade name that is set out in the Compendium or the Grades Document.
Exception — subsection (1)
(2) Subsection (1) does not apply in respect of
- (a) frozen gutted Pacific salmon;
- (b) fresh fruits or vegetables that are exported;
- (c) fresh blueberries, fresh cantaloupes, fresh crabapples, fresh cranberries, fresh field rhubarb and fresh strawberries;
- (d) if they are in a hermetically sealed package, mixed vegetables (macédoine), stewed tomatoes, tomato puree, tomato pulp, tomato paste, tomato ketchup and tomato chili sauce;
- (e) a beef carcass, or a carcass side, hind quarter, front quarter, primal cut or sub-primal cut of a beef carcass that has been imported, in the following cases:
- (i) if it is prepackaged, its container is labelled with the expression “Ungraded Beef” or “bœuf non classifié”, and
- (ii) if it is not prepackaged, it is accompanied by documents for presentation to an inspector or grader that show that it has not been graded; and
- (f) a beef carcass, or a carcass side, hind quarter, front quarter, primal cut or sub-primal cut of a beef carcass, that is sent or conveyed from one province to another or exported, in the following cases:
- (i) if it is prepackaged, its container is labelled with the expression “Ungraded Beef” or “bœuf non classifié”, and
- (ii) if it is not prepackaged, it is accompanied by documents for presentation to an inspector or grader that show that it has not been graded.
Exception — paragraphs (1)(b) and (c)
(3) Paragraphs (1)(b) and (c) do not apply in respect of
- (a) any processed fruit or vegetable product that does not meet the requirements of the Regulations with respect to grade and that is sent or conveyed from one province to another, if it is labelled with the words “Substandard” or “sous-régulier”;
- (b) honey that does not meet the requirements of the Regulations with respect to grade and that is sent or conveyed from one province to another, if it is labelled with the words “Substandard” or “sous-régulier”; and
- (c) honey that does not meet the requirements of the Regulations with respect to grade and that is exported, if the information on the label or the container with respect to the quality of the honey is not false, misleading or deceptive or is not likely to create an erroneous impression.
Exception — paragraph (1)(c)
(4) Paragraph 1(c) does not apply in respect of
- (a) a food that is imported, if the Compendium indicates that it must be labelled with a grade designation that is established by the foreign state of origin and the imported food is labelled with that grade designation rather than with a grade name, and the grade name must be shown in accordance with sections 205, 206 and 312 as if it were a grade name;
- (b) prepackaged fresh fruits or vegetables that are imported, sold in their original container and labelled with a grade designation that is established by the foreign state of origin, if they meet the requirements of the foreign state in respect of that grade designation and those requirements are substantially equivalent to any requirements that apply under these Regulations;
- (c) imported prepackaged fresh fruits or vegetables, other than consumer prepackaged fresh fruits or vegetables, if they are graded and meet the requirements of the Compendium in respect of that grade;
- (d) a beef carcass, or a carcass side, hind quarter, front quarter, primal cut or sub-primal cut of a beef carcass, that is not prepackaged and that is imported if
- (i) it is graded and labelled in accordance with the requirements, in respect of grades of beef carcasses, that are established by the foreign state of origin, or
- (ii) it is accompanied by documents for presentation to an inspector or grader that show the grade designation that is established by the foreign state of origin; and
- (e) a beef carcass, or a carcass side, hind quarter, front quarter, primal cut or sub-primal cut of a beef carcass, that is not prepackaged and does not bear a grade name, and that is sent or conveyed from one province to another, if it is accompanied by documents, for presentation to an inspector or grader, that show its grade name.
Optional grading
307 The following foods for which grades are prescribed by these Regulations, if they are graded and sent or conveyed from one province to another, or imported or exported, must meet the requirements that are set out in the Compendium or the Grades Document in respect of the applicable grade of that food and must be labelled, in accordance with the Compendium or Grades Document, with the applicable grade name that is set out in the Compendium or Grades Document:
- (a) a food referred to in paragraphs 306(2)(a), (c) and (d);
- (b) a dairy product, except for a dairy product that is exported;
- (c) a bison carcass, ovine carcass or veal carcass; and
- (d) a poultry carcass that is dressed or partially dressed.
Authorized application or use
308 (1) Subject to subsection (2), a licence holder is authorized to apply a grade name to, and use a grade name in connection with, a food that is identified in their licence if
- (a) the food meets the requirements of paragraphs 8(1)(a) to (d);
- (b) the food meets the requirements that are set out in the Compendium or the Grades Document in respect of the applicable grade of that food;
- (c) the food complies with any standards that are set out in the Standards of Identity Document;
- (d) in the case of a dairy product, an egg, fish, a processed fruit or vegetable product, honey or maple syrup, the food has been graded by a licence holder;
- (e) in the case of a livestock carcass or poultry carcass that is dressed or partially dressed, the food has been graded by a grader; and
- (f) the food is packaged and labelled in accordance with these Regulations.
Livestock carcass or poultry carcass
(2) In the case of a livestock carcass or a poultry carcass that is dressed or partially dressed, only the persons who are referred to in the Compendium or the Grades Document are authorized, under the circumstances set out in the Compendium or the Grades Document, to apply a grade name to, or use a grade name in connection with, a food in accordance with this Part.
Imported foods — no prescribed grade name
309 A food that is imported and in respect of which no grade name is prescribed by these Regulations may be labelled with the grade designation that is established by the foreign state of origin if
- (a) the food meets the requirements for the grade designation that are established by that foreign state;
- (b) the food is labelled in accordance with these Regulations; and
- (c) the name of that foreign state of origin is clearly indicated on the label.
Authorized reproduction
310 The following persons are authorized to reproduce a grade name:
- (a) printers of labels or manufacturers of packages, if the labels or packages that bear the grade name are provided to a person who is authorized to apply or use the grade name under section 308;
- (b) publishers of documents on the subject of a food that is graded;
- (c) publishers of documents that advertise a food that is graded; and
- (d) manufacturers of grade stamp applicators or grade rollers, if the grade stamps applicators or grade rollers are provided to a grader.
Advertising or sale
311 Any person is authorized to use a grade name in the advertising or sale of a food if the food is labelled with the grade name in accordance with these Regulations.
DIVISION 4
Packaging and Labelling
SUBDIVISION A
General
Labelling of grade name — consumer prepackaged food
312 In the case of a consumer prepackaged food, the grade name must be shown
- (a) on the principal display panel or in the manner set out in the Compendium; and
- (b) in characters of the height that is required by another provision in this Division or, if there is no such provision, in characters of at least the minimum character height that is set out in column 2 of Schedule 6 for the area of a principal display surface that is set out in column 1.
Illustration of grade name
313 A grade name that is applied to a beef carcass, bison carcass, ovine carcass, veal carcass, poultry carcass that is dressed or partially dressed, dairy product or egg must be shown as illustrated in the Compendium or the Grades Document.
SUBDIVISION B
Eggs
Grade name — prepackaged eggs
314 (1) The grade name on prepackaged eggs must be shown
- (a) in the case of a tray with an overwrap or an egg carton, on the top of the tray or egg carton; and
- (b) in the case of a container, other than a tray with an overwrap or an egg carton, in a central location on the container, except the top or the bottom.
Exception — paragraph (1)(a)
(2) The grade name is not required to be shown on trays with an overwrap or on egg cartons that are packaged inside of a second container if the grade name is shown on the second container and the second container is sent or conveyed to an establishment where eggs are graded, packaged and labelled by a licence holder.
Exception — paragraph (1)(b)
(3) If consumer prepackaged eggs in a container other than a tray with an overwrap or an egg carton are packaged inside of a second container, the grade name is not required to be shown on the second container if the grade name is readily discernable and legible without opening the second container and the grade name is not obscured by the second container.
Type size
315 The grade name on prepackaged eggs must be shown in characters that are at least the following height:
- (a) in the case of a tray with an overwrap or an egg carton of eggs graded Canada A or Canada B, 1.5 mm for the word “Canada” and 3 mm for “A” or “B”;
- (b) in the case of a tray with an overwrap or an egg carton of eggs graded Canada C or Canada Nest Run, 1.5 mm;
- (c) in the case of a container, other than a tray with an overwrap or an egg carton, that contains eggs graded Canada A or Canada B, 6 mm for the word “Canada” and 13 mm for “A” or “B”; and
- (d) in the case of a container, other than a tray with an overwrap or an egg carton, that contains eggs graded Canada C or Canada Nest Run, 13 mm.
Eggs — Canada A
316 Eggs that are graded Canada A must be labelled with any applicable size designation that is set out in the Compendium. The size designation must be shown on the container in close proximity to the grade name.
SUBDIVISION C
Fish
Prepackaged fish
317 Prepackaged fish that is sent or conveyed from one province to another or that is imported or exported must be labelled with any applicable class and size designation that is set out in the Compendium. The class name or size designation must be shown in close proximity to the grade name and in characters that are at least 3.2 mm in height.
Second container
318 If consumer prepackaged fish that is labelled in accordance with these Regulations is placed inside of a second container and the resulting product is consumer prepackaged fish, the second container is not required to be labelled with the grade name.
Type size
319 The grade name of prepackaged fish must be shown in characters that are at least 3.2 mm in height if the declaration of net quantity is 900 g or less.
SUBDIVISION D
Fresh Fruits or Vegetables
Grade name — prepackaged fresh fruits or vegetables
320 (1) The grade name of prepackaged fresh fruits or vegetables, other than consumer prepackaged fresh fruits or vegetables, must be shown
- (a) on any surface of the container, except the bottom; and
- (b) in characters of at least the minimum character height that is set out in column 2 of Schedule 6 for the area of a principal display surface that is set out in column 1.
Exception — type size
(2) Despite subsection (1), the grade name of prepackaged fresh fruits or vegetables, other than consumer prepackaged fresh fruits or vegetables, may, if the container is a reusable plastic container, be in characters that are at least 1.6 mm in height.
Exception — second container
(3) If prepackaged fresh fruits or vegetables that are labelled in accordance with these Regulations are placed inside of a second container and the resulting product is prepackaged fresh fruits or vegetables, other than consumer prepackaged fresh fruits or vegetables, the second container is not required to be labelled with the grade name if the grade name is readily discernible and legible without having to open the second container and the grade name is not obscured by the second container.
Size designation
321 Fresh fruits or vegetables that are sent or conveyed from one province to another or imported must be labelled with the applicable size designation that is set out in the Compendium, if any. The size designation must
- (a) be shown in close proximity to the grade name;
- (b) in the case of prepackaged fresh fruits or vegetables, other than consumer prepackaged fresh fruits or vegetables,
- (i) if their container is a reusable plastic container, be shown in characters that are at least 1.6 mm in height, or
- (ii) if their container is not a reusable plastic container, be shown in characters of at least the minimum character height that is set out in paragraph 320(1)(b) for the grade name; and
- (c) in the case of consumer prepackaged fresh fruits or vegetables, be shown in characters of at least the minimum character height that is set out in column 2 of Schedule 6 for the area of a principal display surface that is set out in column 1.
SUBDIVISION E
Processed Fruit or Vegetable Products
Size designation
322 (1) A processed fruit or vegetable product that is sent or conveyed from one province to another or that is imported or exported must be labelled with any applicable size designation that is set out in the Compendium. The size designation must be shown in close proximity to the grade name in characters that are at least 1.6 mm in height.
Exception
(2) Despite subsection (1), green or wax beans, peas, lima beans, asparagus tips or spears, whole white potatoes, whole carrots or cut carrots-whole style that are in a hermetically sealed package and have not been size graded or that are not all of the same size may be labelled, as the case may be, with the expression
- (a) “Ungraded as to Size” or “non calibré”; or
- (b) “Assorted Sizes” or “grosseurs assorties” or “Mixed Sizes” or “grosseurs mixtes”.
SUBDIVISION F
Honey
Grade name — prepackaged honey
323 The grade name of prepackaged honey, other than consumer prepackaged honey, must be shown
- (a) on at least one side or one end of the container; and
- (b) in characters that are at least 9.5 mm in height.
Colour class
324 Honey that is sent or conveyed from one province to another or that is imported or exported must be labelled with any applicable colour class that is set out in the Compendium. The colour class must be shown on the container, in close proximity to the grade name,
- (a) in the case of consumer prepackaged honey, in characters that are of at least the minimum character height that is set out in column 2 of Schedule 6 for the area of a principal display surface that is set out in column 1; or
- (b) in the case of prepackaged honey other consumer prepackaged honey, in the manner set out in paragraphs 323(a) and (b).
SUBDIVISION G
Maple Syrup
Colour class
325 Maple syrup that is graded Canada Grade A and is sent or conveyed from one province to another or exported, or that is graded Grade A and is imported, must be labelled with the applicable colour class that is set out in the Compendium. The colour class must be shown on the container in characters of at least the minimum character height that is set out in column 2 of Schedule 6 for the area of a principal display surface that is set out in column 1.
SUBDIVISION H
Livestock Carcasses
Prepackaged cut of beef
326 A grade name that is applied to a prepackaged primal cut or sub-primal cut of a beef carcass must correspond to the grade of the beef carcass from which it is cut.
Beef — Canada AAA
327 A cut from a beef carcass that is graded Canada AAA and that is exported in a container may be labelled with the expression “Canada Choice” or “Choix Canada” instead of the grade name.
Livestock carcass — removal of marking
328 (1) A grade stamp, roller brand or yield stamp must not be removed from a livestock carcass or a primal cut of a livestock carcass unless the removal is at the direction of and under the supervision of a grader or the livestock carcass or primal cut is being trimmed for further processing.
Removal of marked fat
(2) If fat that is marked with a grade stamp, roller brand or yield stamp is removed from a livestock carcass or a primal cut, the fat must be disposed of under a grader’s supervision unless the fat is
- (a) reapplied to the same livestock carcass or primal cut from which it was removed; or
- (b) applied, under a grader’s supervision, to another livestock carcass or primal cut that bears the same grade stamp, roller brand or yield stamp.
Beef carcass — rib
(3) If the carcass referred to in paragraph (2)(b) is a beef carcass that is graded Canada A, Canada AA, Canada AAA or Canada Prime, the fat must be applied to the rib of the carcass.
Livestock carcass — additional marks
329 A livestock carcass or primal cut of a livestock carcass that is marked with a grade stamp, roller brand or yield stamp may bear another mark only if
- (a) the mark is shown no more than once on the livestock carcass or once on each primal cut;
- (b) the mark is shown alone or in combination with a date;
- (c) the size in height and width of the mark or, if a date is shown, of the mark and date in combination does not exceed 76 mm; and
- (d) the mark and any date do not touch the grade stamp, the roller brand or the yield stamp.
SUBDIVISION I
Poultry Carcasses
Grade name — poultry carcass
330 (1) The grade name of a poultry carcass must be shown
- (a) in the case of a poultry carcass that is individually packaged, on the part of the package that lies on or over the anterior centre of the breast of the poultry; and
- (b) in the case of a poultry carcass that is not individually packaged, on a tag that is attached to the V of the wishbone of the carcass.
Type size
(2) The grade name of a poultry carcass must be shown in characters that are
- (a) in the case of poultry carcass that is not individually packaged or is in a container labelled “not for further processing” or « aucune transformation ultérieure » and individually wrapped, at least 1.5 mm in height;
- (b) in the case of a consumer prepackaged poultry carcass graded Canada A or Canada C that has a net weight of 1 kg or less, at least 3 mm in height;
- (c) in the case of a consumer prepackaged poultry carcass graded Canada A or Canada C that has a net weight of more than 1 kg but not more than 5 kg, at least 6 mm in height;
- (d) in the case of a consumer prepackaged poultry carcass graded Canada A or Canada C that has a net weight of more than 5 kg, at least 9 mm in height;
- (e) in the case of a consumer prepackaged poultry carcass graded Canada Utility that has a net weight of 5 kg or less, at least 3 mm in height;
- (f) in the case of a consumer prepackaged poultry carcass graded Canada Utility that has a net weight of more than 5 kg, at least 5 mm in height; and
- (g) in the case of a prepackaged poultry carcass, other than a consumer prepackaged poultry carcass, at least 6 mm in height.
Packaging in same container
331 Only graded poultry carcasses that are dressed or partially dressed and that have the same common name may be packaged in the same container.
DIVISION 5
Conditions for Grading of Certain Foods
SUBDIVISION A
Grading of Eggs
Conditions for grading
332 (1) A licence holder may grade an egg only if the egg
- (a) is edible;
- (b) does not emit an abnormal odour;
- (c) is not mouldy;
- (d) has not been in an incubator;
- (e) does not have any internal defect; and
- (f) is of a usual colour.
Exception
(2) Despite paragraph (1)(e), a licence holder may grade and apply the grade name Canada C to an egg that has a particle of the oviduct or a blood spot neither of which exceeds 3 mm in diameter.
Ungraded eggs
333 (1) Ungraded eggs that are received at an establishment where eggs are graded by a licence holder must be graded and labelled with the applicable grade name that is set out in the Compendium or, if they do not meet the requirements in respect of any grade that are set out in these Regulations, they must be rejected.
Rejected eggs
(2) Eggs that are rejected must be destroyed or be placed in a container that is labelled with the words “Rejects” and “rejetés”.
SUBDIVISION B
Grading of Livestock Carcasses
Request for grading
334 A grader may grade a livestock carcass in an establishment that is identified in a licence or in a provincial establishment if one of the following persons has requested in writing that the carcass be graded:
- (a) any person who is in authority in the establishment;
- (b) any producer; or
- (c) the person who is in possession of the livestock carcass.
Conditions for grading
335 A grader may grade a livestock carcass if
- (a) the carcass bears a meat inspection stamp or, in the case of an imported beef carcass, the official inspection mark of the foreign state of origin;
- (b) the grading takes place
- (i) in the case of a bison carcass or ovine carcass, in the establishment that is identified in a licence, or the provincial establishment, where the animal was slaughtered,
- (ii) in the case of a veal carcass, in the establishment that is identified in a licence, or the provincial establishment, where the animal was slaughtered or where the carcass was divided into primal cuts or sub-primal cuts, or
- (iii) in the case of a beef carcass, in the establishment that is identified in a licence, or the provincial establishment, where the animal was slaughtered or in the establishment that is identified in a licence where the carcass was divided into primal cuts or sub-primal cuts;
- (c) the carcass has been weighed by a weighmaster using a scale that is approved under section 3 of the Weights and Measures Act;
- (d) the carcass is presented for grading
- (i) at a grading stand where the lighting intensity, measured at the level of the grading stand, is at least 1 000 lx, or
- (ii) in a cooler where the lighting intensity, measured at the loin level of the carcass, is at least 200 lx;
- (e) at least 10 minutes before grading,
- (i) in the case of a beef carcass or bison carcass, an employee of the establishment performs, to the satisfaction of the grader, a knife-rib on the carcass, or
- (ii) in the case of a veal carcass, an employee of the establishment performs, to the satisfaction of the grader, a cut on the lean of the brisket to enable the grader to determine the colour reading of the veal carcass;
- (f) the establishment has adequate equipment and facilities for weighing and grading livestock carcasses; and
- (g) the grading equipment is functioning properly and is accurate.
Adequate facilities
336 (1) If more than 400 livestock carcasses are graded per hour in an establishment that is identified in a licence or in a provincial establishment, more than one grading stand is required for the purposes of paragraph 335(f).
Grading stand — requirements
(2) For the purposes of paragraph 335(f), a grading stand must be easily adjustable for height and must
- (a) if the rate of grading is 150 carcasses per hour or less, be at least 3 m long and 2 m wide;
- (b) if the rate of grading is more than 150 carcasses per hour but not more than 300 carcasses per hour, be at least 4 m long and 2 m wide; and
- (c) if the rate of grading is more than 300 carcasses per hour, be at least 5 m long and 2 m wide.
Weighing before trimming
337 A livestock carcass that is graded must be weighed before it is trimmed, unless an inspector or grader directs that it be trimmed before it is weighed.
SUBDIVISION C
Grading of Poultry Carcasses
Conditions for grading — dressed carcass
338 (1) A grader may grade a poultry carcass that has been dressed if
- (a) the carcass is from poultry slaughtered in an establishment that is identified in a licence or in a provincial establishment;
- (b) the carcass has been inspected under the Act or under an Act of a province that regulates the inspection of poultry carcasses;
- (c) in the case of a chilled poultry carcass, the flesh or skin is not dried out;
- (d) the carcass is not discoloured from insufficient bleeding;
- (e) the carcass has no more than one heart, liver, gizzard and neck packed with it or inserted into it;
- (f) in the case of a poultry carcass that weighs more than 900 g, the breast bone is intact; and
- (g) the carcass has not been basted or stuffed.
Conditions for grading — partially dressed carcass
(2) A grader may grade a poultry carcass that has been partially dressed if
- (a) the carcass meets the requirements that are set out in paragraphs (1)(a) to (g);
- (b) the carcass has been eviscerated;
- (c) the epidermis has been removed from the feet and shanks;
- (d) the claws have been removed;
- (e) the head, if present, is wrapped; and
- (f) the beak, if present, is clean.
Grading in an establishment
(3) A poultry carcass must not be graded in any place other than in an establishment that is identified in a licence or in a provincial establishment.
DIVISION 6
Grading Certificates
Conditions for issuance
339 (1) A grader — or a licence holder, the operator of a provincial establishment or a marketing agency under the direction of a grader — may issue a grading certificate in respect of a livestock carcass or a lot of livestock carcasses if
- (a) at the time of delivery of the food animal or lot of food animals to an establishment that is identified in a licence or to a provincial establishment for slaughter, the producer has
- (i) requested the certificate in writing,
- (ii) identified each animal that is intended to be slaughtered with an identification code, and
- (iii) completed and filed with any person who is in authority in the establishment a list that associates each identification code with the producer; and
- (b) after slaughter, the identification code of each slaughtered animal is retained on or transferred to the livestock carcass by any person who is in authority in the establishment.
Contents of certificate
(2) The grading certificate must be signed by the grader and include the following information:
- (a) the name and address of the producer;
- (b) the name of any person who is acting on behalf of the producer;
- (c) the name and address of the establishment where the livestock carcasses were graded;
- (d) the certificate number;
- (e) the date of slaughter;
- (f) for each livestock carcass,
- (i) its identification code,
- (ii) its warm weight as determined by a weighmaster, and
- (iii) its grade;
- (g) in the case of a lot of livestock carcasses,
- (i) the number of livestock carcasses per grade or per yield class, and
- (ii) the number of livestock carcasses that have been condemned;
- (h) in the case of a grading certificate that is issued in respect of a beef carcass that is graded Canada A, Canada AA, Canada AAA or Canada Prime, the yield of the beef carcass;
- (i) in the case of a grading certificate that is issued in respect of a beef carcass or bison carcass, an indication, for each carcass, of its age, meat colour, firmness, and, if any,
- (i) its musculature,
- (ii) its marbling, specifically, the amount, size and distribution of intramuscular fat deposits in the longissimus muscles,
- (iii) its fat colour or texture,
- (iv) its fat measurement, and
- (v) its pronounced masculinity;
- (j) in the case of a grading certificate that is issued in respect of a lamb carcass, as defined in the Compendium, for each carcass,
- (i) the fat measurement,
- (ii) the score for musculature of each primal cut and the average score for musculature,
- (iii) in the case of a carcass that is graded Canada AAA, the yield, and
- (iv) an indication of any musculature demerits, meat colour demerits or fat colour demerits that were assigned to the carcass; and
- (k) in the case of a grading certificate that is issued in respect of a mutton carcass, as defined in the Compendium, the fat measurement of the carcass.
Recording of information
(3) The information referred to in subsection (2) may be recorded on the grading certificate by the licence holder, the operator or the marketing agency referred to in subsection (1).
PART 13
Organic Products
DIVISION 1
Interpretation
Definitions
340 The following definitions apply in this Part.
aquaculture product has the same meaning as in CAN/CGSB-32.312. (produit aquacole)
CAN/CGSB-32.310 means the Canadian General Standards Board standard CAN/CGSB-32.310, entitled Organic production systems — General principles and management standards, as amended from time to time. (norme CAN/CGSB-32.310)
CAN/CGSB-32.311 means the Canadian General Standards Board standard CAN/CGSB-32.311, entitled Organic production systems — Permitted substances lists, as amended from time to time. (norme CAN/CGSB-32.311)
CAN/CGSB-32.312 means the Canadian General Standards Board standard CAN/CGSB-32.312, entitled Organic production systems — Aquaculture — General principles, management standards and permitted substances lists, as amended from time to time. (norme CAN/CGSB-32.312)
certification body means any person who is accredited as a certification body under section 361 or 363 and who is responsible for the organic certification of food commodities and for the certification of packaging or labelling of organic products. (organisme de certification)
conformity verification body means any person who, having complied with the requirements that are set out in ISO/IEC 17011, has entered into an agreement with the Agency under subsection 14(1) of the Canadian Food Inspection Agency Act to assess, recommend for accreditation and monitor certification bodies. (organisme de vérification de la conformité)
ISO/IEC 17011 means the International Organization for Standardization standard ISO/IEC 17011, entitled Conformity assessment — General requirements for accreditation bodies accrediting conformity assessment bodies, as amended from time to time. (norme ISO/IEC 17011)
ISO/IEC 17065 means the International Organization for Standardization standard ISO/IEC 17065, entitled Conformity assessment — Requirements for bodies certifying products, processes and services, as amended from time to time. (norme ISO/IEC 17065)
Definition food commodity in Act
341 (1) For the purposes of paragraph (c) of the definition food commodity in section 2 of the Act, the following are prescribed food commodities:
- (a) feed as defined in section 2 of the Feeds Act; and
- (b) seed as defined in section 2 of the Seeds Act.
Feed
(2) For the purposes of paragraph (1)(a), a reference to “livestock” in the definition feed in section 2 of the Feeds Act must be read to include livestock that is an aquaculture product.
Exemption
(3) A food commodity that is not included in paragraph (a) or (b) of the definition food commodity in section 2 of the Act is exempted from the application of any provision of the Act and of these Regulations that is not necessary to give effect to this Part. For greater certainty, the exemption does not include section 6 of the Act.
DIVISION 2
Packaging and Labelling
Packaging and labelling
342 The packaging and labelling of an organic product that is to be sent or conveyed from one province to another may only be conducted by a person who holds a certificate that is granted under section 345 or 348.
DIVISION 3
Percentage of Organic Products
Determination of percentage of organic products
343 The percentage of the contents of a multi-ingredient food commodity that are organic products must be determined in accordance with CAN/CGSB-32.310.
DIVISION 4
Certification
SUBDIVISION A
Organic Certification of Food Commodities
Application for organic certification
344 (1) Any person who wishes to obtain the organic certification of a food commodity must apply in writing to a certification body.
Contents of application
(2) The application must include
- (a) the name of the food commodity;
- (b) a statement that sets out the substances and materials that are used to conduct any activities with respect to the food commodity and that describes the manner in which those substances and materials are used;
- (c) a document that sets out in detail the methods that are used by the applicant, or by a person acting on behalf of the applicant, to conduct any activities with respect to the food commodity and the control mechanisms that are in place to ensure that those methods meet the requirements that are set out
- (i) in the case of an aquaculture product, in CAN/CGSB-32.312, and
- (ii) in the case of a food commodity other than an aquaculture product, in CAN/CGSB-32.310;
- (d) in the case where a person packages and labels the food commodity on behalf of the applicant, the name of the person and a copy of the certificate referred to in subsection 348(2) that they hold; and
- (e) in the case of a multi-ingredient food commodity, a statement that sets out its composition and the percentage of its contents that are organic products.
Time of application
(3) In the case of an application for the organic certification of a food commodity, the application must be filed within 12 months before the day on which the food commodity is expected to be sold or, in the case of an application for the organic certification of any of the following food commodities, at least 15 months before that day:
- (a) maple products;
- (b) field crops or crops that are grown in greenhouses with an in-ground permanent soil system;
- (c) wild crops within the meaning of CAN/CGSB-32.312; and
- (d) aquaculture products with a production cycle of more than 12 months.
Certification
345 (1) A certification body must conduct an on-site verification and certify a food commodity as organic if it determines that
- (a) the substances and materials that are used to conduct activities in respect of the food commodity are set out and used in the manner described
- (i) in the case of an aquaculture product, in CAN/CGSB-32.312, and
- (ii) in the case of a food commodity other than an aquaculture product, in CAN/CGSB-32.310 or CAN/CGSB-32.311;
- (b) the methods that are used by the applicant, or by a person acting on behalf of the applicant, to conduct activities in respect of the food commodity and the control mechanisms that are in place meet the requirements and comply with the general principles respecting organic production that are set out
- (i) in the case of an aquaculture product, in CAN/CGSB-32.312, and
- (ii) in the case of a food commodity other than an aquaculture product, in CAN/CGSB-32.310;
- (c) in the case where a person packages and labels the food commodity on behalf of the applicant, the person holds a certificate referred to in subsection 348(2);
- (d) in the case of a multi-ingredient food commodity, at least 70% of its contents are organic products and its composition meets the requirements that are set out in CAN/CGSB-32.310; and
- (e) the information submitted in the application is complete, truthful and not misleading.
Certificate
(2) The certification body must provide the applicant with a certificate that confirms the organic certification of the food commodity and that indicates the name of the food commodity, whether CAN/CGSB-32.310 or CAN/ CGSB-32.312 is applicable, and, in the case of a multi-ingredient food commodity, whether at least 70% of its contents are organic products or whether at least 95% of its contents are organic products.
Requirement to provide information
346 (1) The holder of the certificate referred to in subsection 345(2) must provide to the certification body the information that is set out in subsection 344(2) once every 12-month period beginning on the day on which the certificate is issued and no later than the day that is six months before the end of that period.
On-site verification
(2) After receiving the information from the certificate holder under subsection (1), the certification body must, no later than the end of the 12-month period referred to in that subsection, conduct an on-site verification to determine whether the requirements that are set out in subsection 345(1) are met.
SUBDIVISION B
Certification of Packaging and Labelling
Application for certification
347 (1) Any person who wishes to package or label an organic product, other than a product in respect of which they hold a certificate granted under section 345, must apply in writing to a certification body for certification of the activity.
Contents of application
(2) The application must include
- (a) an indication of the type of organic product;
- (b) a statement that sets out the substances and materials that the applicant will use to package or label the organic product and that describes the manner in which those substances and materials will be used; and
- (c) a document that sets out in detail the methods that the applicant will use to package or label the organic product and the control mechanisms that the applicant will put in place to ensure that those methods meet the requirements that are set out
- (i) in the case of an aquaculture product, in CAN/CGSB-32.312, and
- (ii) in the case of a food commodity other than an aquaculture product, in CAN/CGSB-32.310.
Certification
348 (1) A certification body must conduct an on-site verification and certify the activity in respect of the packaging or labelling of an organic product if it determines that
- (a) the substances and materials that are used by the applicant for packaging or labelling are set out and used in the manner described
- (i) in the case of an aquaculture product, in CAN/CGSB-32.312, and
- (ii) in the case of a food commodity other than an aquaculture product, in CAN/CGSB-32.310 or CAN/CGSB-32.311;
- (b) the methods that are used by the applicant for packaging or labelling and the control mechanisms that are in place meet the requirements and comply with the general principles respecting organic production that are set out
- (i) in the case of an aquaculture product, in CAN/CGSB-32.312, and
- (ii) in the case of a food commodity other than an aquaculture product, in CAN/CGSB-32.310.
Certificate
(2) The certification body must provide the applicant with a certificate that confirms the certification of the packaging or labelling of the organic product and that indicates the type of organic product to which it applies and the period of validity referred to in subsection (3).
Period of validity
(3) The certification of the packaging or labelling of an organic product is valid for 12 months beginning on the day on which it is granted under subsection (1).
SUBDIVISION C
Suspension and Cancellation
Suspension
349 (1) Subject to subsection (2), the certification body must suspend a certification that is granted under section 345 or 348 if
- (a) the holder of the certificate does not comply with any provision of the Act or this Part;
- (b) the substances or materials that are used are other than those that are set out
- (i) in the case of an aquaculture product, in CAN/CGSB-32.312, and
- (ii) in the case of a food commodity other than an aquaculture product, in CAN/CGSB-32.310 or CAN/CGSB-32.311;
- (c) the food commodity comes into contact with a substance or material other than one that is set out
- (i) in the case of an aquaculture product, in CAN/CGSB-32.312, and
- (ii) in the case of a food commodity other than an aquaculture product, in CAN/CGSB-32.310 or CAN/CGSB-32.311;
- (d) the substances or materials that are used are set out but are not used in the manner described,
- (i) in the case of an aquaculture product, in CAN/CGSB-32.312, and
- (ii) in the case of a food commodity other than an aquaculture product, in CAN/CGSB-32.310 or CAN/CGSB-32.311;
- (e) the methods that are used do not meet the requirements or do not comply with the general principles respecting organic production that are set out
- (i) in the case of an aquaculture product, in CAN/CGSB-32.312, and
- (ii) in the case of a food commodity other than an aquaculture product, in CAN/CGSB-32.310; or
- (f) in the case of a multi-ingredient food commodity, less than 70% of its contents are organic products.
Conditions
(2) The certification body must not suspend a certification unless the holder of the certificate
- (a) was provided with a written report that sets out the grounds for the suspension and the period within which corrective action must be taken in order to avoid the suspension; and
- (b) failed to take corrective action within that period or, if the certification body granted an extension at the written request of the holder, within any later period specified by the certification body.
Extension of period
(3) The certification body may grant an extension of the period in which corrective action must be taken only once.
Written notice
(4) The certification body must notify the holder of the certificate in writing of the suspension and the date on which it takes effect.
Duration of suspension
(5) The suspension of a certification must be lifted if the certification body determines that corrective action has been taken.
Cancellation
350 (1) The certification body must cancel a certification if
- (a) the holder of the certificate fails to take corrective action within 30 days after the day on which the certification was suspended;
- (b) the holder of the certificate was not in compliance with section 15 of the Act in respect of the application made under section 344 or 347 or at any time during the period of validity of the certification; or
- (c) while the certification is suspended,
- (i) in the case of a certification that was granted under section 345, the holder of the certificate
- (A) sends or conveys from one province to another a food commodity that is labelled with an expression that is referred to in subsection 353(1) or (2),
- (B) sends or conveys from one province to another a food commodity that has on it the product legend that is set out in Schedule 9 or a food commodity in connection with which that product legend is used,
- (C) applies or attaches to a food commodity a label that bears an expression that is referred to in subsection 353(1) or (2) or uses such an expression in the advertisement of a food commodity,
- (D) applies the product legend that is set out in Schedule 9 to, or uses it in connection with, a food commodity, or
- (E) conducts any activity with respect to a food commodity that is identified in the certificate, and
- (ii) in the case of a certification that was granted under section 348, the holder of the certificate packages or labels an organic product.
- (i) in the case of a certification that was granted under section 345, the holder of the certificate
Conditions
(2) The certification body must not cancel a certification unless the holder of the certificate was notified in writing of the grounds for the cancellation and was provided with an opportunity to be heard in respect of the cancellation.
Written notice
(3) The certification body must notify the holder of the certificate in writing of the cancellation and the date on which it takes effect.
SUBDIVISION D
General
Documents
351 The holder of a certificate must prepare, keep and maintain the documents that are set out in the following standards, in accordance with those standards:
- (a) in the case of an aquaculture product, CAN/ CGSB-32.312; and
- (b) in the case of a food commodity other than an aquaculture product, CAN/CGSB-32.310.
Changes affecting certification
352 The holder of a certificate must immediately notify the certification body in writing of any change that could affect the certification and of any complaint that they receive in relation to the organic integrity of the organic product referred to in the certification.
DIVISION 5
Labelling and Advertising
Expressions
353 (1) The expressions “organic” or “biologique” or “organique”, “organically grown” or “cultivé biologiquement”, “organically raised” or “élevé biologiquement” and “organically produced” or “produit biologiquement” and any similar expressions, including abbreviations of, symbols for and phonetic renderings of those expressions, may be shown on the label or used in the advertisement of a food commodity that is sent or conveyed from one province to another if
- (a) the food commodity is an organic product; and
- (b) in the case of a multi-ingredient food commodity, at least 95% of its contents are organic products.
Expression “Organic ingredients”
(2) Despite subsection (1), if a multi-ingredient food commodity is an organic product but less than 95% of its contents are organic products, it may be labelled with or advertised using the expression “organic ingredients” or “d’ingrédients biologiques” if that expression is
- (a) immediately preceded by the percentage of its contents that are organic products, rounded down to the nearest whole number; and
- (b) in characters of the same height and prominence as the words, numbers, signs or symbols that indicate that percentage.
Multi-ingredient food commodities
(3) Despite subsection (1), the list of ingredients that is shown on the label of a multi-ingredient food commodity that is not an organic product may indicate which of the ingredients are organic products.
Additional information
354 If an expression that is referred to in subsection 353(1) or (2) is shown on the label of a food commodity, the label must also bear
- (a) in the case of a food commodity that is sent or conveyed from one province to another, the name of the certification body that certified the food commodity as organic;
- (b) in the case of a food commodity that is imported, the name of the certification body or the name of the entity accredited by a foreign state referred to in subparagraph 357(1)(a)(ii) or (iii) that certified the food commodity as organic;
- (c) in the case of a multi-ingredient food commodity that is sent or conveyed from one province to another or that is imported, the organic contents that are identified as organic in its list of ingredients; and
- (d) in the case of a food commodity that is imported and on whose label the product legend that is set out in Schedule 9 is applied, the expression “Product of” or “produit de” immediately preceding the name of the foreign state of origin or the word “Imported” or “importé” in close proximity to that product legend.
Official languages
355 (1) Subject to subsection (2), the expressions that are referred to in subsections 353(1) and (2) and paragraph 354(d) and the information that is referred to in paragraph 354(c) must be shown on the label of a food commodity in both official languages.
Exception
(2) Those expressions and that information may be shown on the label of a food commodity in only one official language if the food commodity is any of the following:
- (a) a feed as defined in section 2 of the Feeds Act;
- (b) a seed as defined in section 2 of the Seeds Act; or
- (c) a food, if subsection B.01.012(3), (7) or (11) of the Food and Drug Regulations allows the required information to be shown in only one official language.
Feed
(3) For the purposes of paragraph (2)(a), a reference to “livestock” in the definition feed in section 2 of the Feeds Act must be read to include livestock that is an aquaculture product.
DIVISION 6
Interprovincial Trade and Import
Interprovincial trade
356 (1) A food commodity that is sent or conveyed from one province to another and that is labelled with or advertised using an expression that is referred to in subsection 353(1) or (2) must
- (a) be an organic product;
- (b) meet the requirements for the use of that expression that are set out in subsection 353(1) or (2), as the case may be; and
- (c) meet the requirements of sections 354 and 355.
Multi-ingredient food commodities
(2) In the case of a multi-ingredient food commodity that is not an organic product and that is sent or conveyed from one province to another, the list of ingredients that is shown on the label of the food commodity may indicate which of the ingredients are organic products.
Import
357 (1) A food commodity that is imported and that is labelled with or advertised using an expression that is referred to in subsection 353(1) or (2) must
- (a) meet one of the following requirements:
- (i) be certified as organic under subsection 345(1),
- (ii) be imported from a foreign state with which the Agency has entered into an agreement or arrangement regarding the import and export of organic products and be certified as organic, in accordance with the agreement or the arrangement, by an entity that is accredited by that foreign state, or
- (iii) be imported from a foreign state with which the Agency has not entered into such an agreement or arrangement, but be certified as organic by an entity that is accredited by a foreign state that is referred to in subparagraph (ii) with the certification being in accordance with the agreement or arrangement referred to in that subparagraph;
- (b) meet the requirements for the use of that expression under subsection 353(1) or (2), as the case may be; and
- (c) meet the requirements of sections 354 and 355.
Multi-ingredient food commodities
(2) In the case of a multi-ingredient food commodity that is not an organic product and that is imported, the list of ingredients that is shown on the label of the food commodity may indicate which of the ingredients are organic products.
Demonstration
(3) The person who imports the organic product must be able to demonstrate that the product meets one of the requirements of paragraph (1)(a) by providing, at the request of the Minister or an inspector, a certificate that confirms the organic certification of the product.
Retention period of certificate
(4) The certificate referred to in subsection (3) must be kept for five years after the day on which the organic product is imported.
DIVISION 7
Product Legend
Definition inspection mark in Act
358 For the purposes of the definition inspection mark in section 2 of the Act, the product legend that is set out in Schedule 9 is prescribed.
Application or use of product legend
359 (1) A person is authorized to apply the product legend that is set out in Schedule 9 to and use it in connection with a food commodity if
- (a) the food commodity is an organic product; and
- (b) in the case of a multi-ingredient food commodity, at least 95% of its contents are organic products.
Advertisement and sale
(2) A person is authorized to advertise and sell a food commodity that has on it the product legend that is set out in Schedule 9, or a food commodity in connection with which that product legend is used, if
- (a) the food commodity is an organic product; and
- (b) in the case of a multi-ingredient food commodity, at least 95% of its contents are organic products.
Application or use — other than food commodity
(3) A person is authorized, for advertisement or information purposes, to apply the product legend that is set out in Schedule 9 to, and use it in connection with, any item to which the Act applies other than a food commodity.
DIVISION 8
Conformity Verification Bodies and Certification Bodies
Application for accreditation
360 Any person who wishes to be accredited as a certification body must apply for the accreditation, in writing, to a conformity verification body and must undergo an assessment, in accordance with ISO/IEC 17011, to verify
- (a) their compliance with ISO/IEC 17065;
- (b) their knowledge with respect to organic certification and that of their employees and of any persons acting on their behalf; and
- (c) the validity of their certification methodology and the validity of the results of that methodology.
Accreditation
361 (1) On the recommendation of a conformity verification body, accompanied by supporting documents, the Minister must accredit the applicant, provide them with an accreditation number and notify them in writing of the period of validity referred to in subsection (2).
Period of validity
(2) The accreditation of a certification body is valid for five years beginning on the day on which the Minister accredits the applicant.
Refusal
362 If the conformity verification body refuses to recommend the applicant’s accreditation, it must send them a written notice by registered mail that states the reasons for the decision and notifies the applicant of their right to make a request, within 30 days after the day on which they receive the notice, to the Minister for a review of the decision. The conformity verification body must also send a copy of the notice to the Minister.
Review
363 The Minister must, at the written request of the applicant, review the decision referred to in section 362 and, if the Minister decides to confirm it, must provide a copy of his or her decision with reasons to the applicant. If the Minister does not confirm the decision, the Minister must accredit the applicant, provide them with an accreditation number and notify them in writing of the period of validity referred to in subsection 361(2).
Suspension
364 (1) Subject to subsection (2), on the recommendation of a conformity verification body, the Minister must suspend the accreditation of a certification body if it does not comply with any provision of the Act, this Part or ISO/IEC 17065.
Conditions
(2) The Minister must not suspend an accreditation unless the certification body
- (a) was provided with a written report that sets out the grounds for the suspension and the period within which corrective action must be taken in order to avoid the suspension; and
- (b) failed to take corrective action within that period or, if the conformity verification body granted an extension at the written request of the certification body, within any later period specified by the conformity verification body.
Extension of period
(3) The conformity verification body may grant an extension of the period in which corrective action must be taken only once.
Written notice
(4) The Minister must notify the certification body in writing of the suspension and the date on which it takes effect.
Provision of lists
(5) The certification body must provide the Minister, within 15 days after the day on which the suspension takes effect, with a list of the holders of the certificates that it has granted and a list of pending applications for certification.
Duration of suspension
(6) The suspension of an accreditation must be lifted if the Minister determines, on the recommendation of the conformity verification body, that corrective action has been taken.
Cancellation
365 (1) On the recommendation of a conformity verification body, the Minister must cancel an accreditation if the certification body
- (a) fails to take corrective action within 30 days after the day on which the accreditation was suspended;
- (b) was not in compliance with section 15 of the Act in respect of the application made under section 360 or at any time during the period of validity of the accreditation; or
- (c) continues, while their accreditation is suspended, to accept applications for certification, to make determinations under subsection 345(1) or 348(1), to suspend certifications under subsection 349(1) or to cancel certifications under subsection 350(1).
Conditions
(2) The Minister must not cancel a certification unless the certification body was notified in writing of the grounds for the cancellation and was provided with an opportunity to be heard in respect of the cancellation.
Written notice
(3) The Minister must notify the certification body in writing of the cancellation and the date on which it takes effect.
PART 14
Seizure and Detention
Detention tag
366 An inspector who seizes and detains a thing under section 25 of the Act must apply or attach a detention tag to it which bears the following:
- (a) the expression “UNDER DETENTION” and the word “RETENU”, in capital letters;
- (b) the detention tag number;
- (c) a description of the thing;
- (d) the reason for the seizure and detention;
- (e) the date of the seizure and detention; and
- (f) the inspector’s name and signature.
Prohibition — removal of detention tag
367 It is prohibited for a person to remove a detention tag from a thing that has been seized and detained unless authorized to do so by an inspector.
Notice of detention
368 (1) As soon as feasible after a thing has been seized and detained, an inspector must provide a notice of detention to its owner or to the person having possession, care or control of it at the time of its seizure.
Content of notice of detention
(2) The notice of detention must indicate that the thing was seized and detained under section 25 of the Act and set out
- (a) the detention tag number;
- (b) a description of the thing;
- (c) the reason for the seizure and detention;
- (d) the date of the seizure and detention;
- (e) the place of the seizure and detention;
- (f) the inspector’s name and signature; and
- (g) a telephone number to call for further information about the seizure and detention.
Storage conditions
369 Anything that is seized must be stored by the person to whom the notice of detention is provided, under storage conditions that are appropriate for its preservation and at the person’s expense.
Notice of release
370 If a thing is released under section 30 of the Act, an inspector must provide a notice of release to the person to whom the notice of detention was provided.
PART 15
Transitional Provisions
18-month delay
371 (1) Subsections 5(2) and 7(2), section 11, paragraphs 15(1)(a) and (b), subsections 18(3) and 19(2) and Parts 4 and 5 do not apply, for the 18-month period that begins on the day on which these Regulations come into force, in respect of foods other than dairy products, eggs, processed egg products, fish, fresh fruits or vegetables, processed fruit or vegetable products, honey, maple products and meat products.
Additional delay — four employees or less
(2) Section 11 and Part 4 do not apply to any person who did not have more than four employees at any one time during the last 12 months of the period referred to in subsection (1) in respect of foods other than dairy products, eggs, processed egg products, fish, fresh fruits or vegetables, processed fruit or vegetable products, honey, maple products and meat products for 12 months after the last day of the period referred to in that subsection.
Additional delay — $100,000 or less
(3) Sections 11 and 45 to 85 do not apply to any person whose gross sales derived from food were $100,000 or less for the last 12 months of the period referred to in subsection (1) in respect of foods other than dairy products, eggs, processed egg products, fish, fresh fruits or vegetables, processed fruit or vegetable products, honey, maple products and meat products for 12 months after the last day of the period referred to in that subsection.
Fresh fruits or vegetables — 12-month delay
372 For the 12-month period that begins on the day on which these Regulations come into force, section 11 and Part 4 do not apply in respect of fresh fruits or vegetables and Part 5 does not apply in respect of fresh fruits or vegetables to any person who grows or harvests fresh fruits or vegetables unless the person is the holder of a licence to conduct an activity in respect of those fresh fruits or vegetables.
Aquaculture products — 24-month delay
373 (1) Part 13 does not apply in respect of aquaculture products — other than seaweed in respect of which a certification has been issued under section 13 of the Organic Products Regulations, 2009 — for the 24-month period that begins on the day on which these Regulations come into force.
Exception
(2) However, during that period, an application referred to in section 344 or 347 may be made in respect of any aquaculture product, including seaweed, and a certification in respect of the aquaculture product may be granted under section 345 or 348. If such a certification is granted, Part 13 applies in respect of any aquaculture product referred to in the certification.
Food commodities deemed to meet applicable requirements
374 (1) A food commodity that, immediately before the day on which these Regulations come into force, meets the requirements that apply in respect of that food commodity under the Consumer Packaging and Labelling Act, the Fish Inspection Act, the Food and Drugs Act, the Meat Inspection Act and the Canada Agricultural Products Act is deemed, as of the day on which these Regulations come into force, to meet the requirements that apply under these Regulations with respect to the manufacturing, preparing, storing, packaging and labelling of that food commodity if those activities are conducted before that day.
Reference to Act
(2) In paragraph 29(1)(a), subparagraph 30(a)(ii) and paragraph 39(c), a reference to “the Act” must be read to include a reference to any provisions of the Consumer Packaging and Labelling Act that apply in respect of a food, the Fish Inspection Act, the Meat Inspection Act, and the Canada Agricultural Products Act, as they read immediately before the coming into force of these Regulations.
Inspection legends
(3) For the purposes of sections 179 to 183, subsection 184(1), section 185, paragraphs 258(a), 282(1)(a) and 287(1)(a), the following are deemed to be an inspection legend that is set out in Figure 1 or 2 of Schedule 2, for the 36-month period that begins on the day on which these Regulations come into force:
- (a) in the case of a meat product, an inspection legend set out in Figure 1, 2 or 3 of Schedule III to the Meat Inspection Regulations, 1990, as they read immediately before the coming into force of force of these Regulations, or such an inspection legend without the registration number if it meets the conditions under subsection 93(3) of those Regulations, as they read immediately before the coming into force of force of these Regulations;
- (b) in the case of a prepackaged processed egg product, the inspection legend set out in Schedule II to the Processed Egg Regulations, as they read immediately before the coming into force of these Regulations; and
- (c) in the case of prepackaged fish, a mark or label with a designation that is referred to in section 28 of the Fish Inspection Regulations, as they read immediately before the coming into force of these Regulations.
Certificates, authorizations, exemptions, certifications and accreditations
375 (1) A certificate, authorization, exemption, certification or accreditation that is set out in column 1 of the table to this section and that is valid immediately before the day on which these Regulations come into force is deemed to have been issued or obtained under the provision of the Act or these Regulations that is set out in column 2.
Period of validity
(2) Subject to subsection (3), unless it is suspended or cancelled under these Regulations, the certificate, authorization, exemption, certification or accreditation remains valid until the end of the period for which it was issued or obtained.
Period of validity — seaweed
(3) An organic certification of seaweed granted under section 13 of the Organic Products Regulations, 2009 ceases to be valid on the expiry of the 24-month period that begins on the day on which these Regulations come into force.
Suspensions
(4) A certificate, authorization, exemption, certification or accreditation that was suspended before the day on which these Regulations come into force and that continues to be suspended on that day is deemed to be suspended under these Regulations.
Applications for certificates, authorizations, exemptions, certifications or accreditations
(5) An application for a certificate, authorization, exemption, certification or accreditation that is set out in column 1 of the table to this section that was made before the day on which these Regulations come into force and in respect of which no decision has been made is deemed to be an application made under these Regulations for the certificate, authorization, exemption, certification or accreditation referred to in the provision of the Act or these Regulations that is set out in column 2.
TABLE
| Item |
Column 1 |
Column 2 |
|---|---|---|
1 |
Certificate issued under section 24 of the Egg Regulations in respect of eggs for export |
Section 48 of the Act |
2 |
Authorization obtained under subsection 29.1(5) of the Meat Inspection Regulations, 1990 |
Subsection 160(3) of these Regulations |
3 |
Exemption obtained under section 2.2 of the Fresh Fruit and Vegetable Regulations |
Subsection 174(2) of these Regulations |
4 |
Authorization obtained under subsection 2.3(2) of the Fresh Fruit and Vegetable Regulations |
Subsection 174(2) of these Regulations |
5 |
Authorization obtained under subsection 29(4) of the Honey Regulations |
Subsection 174(2) of these Regulations |
6 |
Authorization issued under subsection 9.1(5) of the Processed Products Regulations |
Subsection 174(2) of these Regulations |
7 |
Exemption obtained under section 59.2 of the Processed Products Regulations |
Subsection 174(2) of these Regulations |
8 |
Exemption obtained under section 63 of the Processed Products Regulations |
Subsection 174(2) of these Regulations |
9 |
Certification issued under section 13 of the Organic Products Regulations, 2009 |
Section 345 of these Regulations |
10 |
Certification issued under section 15 of the Organic Products Regulations, 2009 |
Section 348 of these Regulations |
11 |
Accreditation issued under section 6 or 8 of the Organic Products Regulations, 2009 |
Section 361 or 363 of these Regulations |
Subsection 36(3) of Consumer Packaging and Labelling Regulations
376 (1) Every exemption referred to in subsection 36(3) of the Consumer Packaging and Labelling Regulations in respect of a test market product that is valid immediately before the day on which these Regulations come into force is deemed to be an exemption granted under subsection 174(2) of these Regulations.
Period of validity
(2) Unless cancelled under these Regulations, the exemption remains valid until the product ceases to be a test market product under subsection 6(6) of the Consumer Packaging and Labelling Regulations, as they read immediately before the day on which these Regulations come into force.
Request for exemption
(3) A notice of intention that is filed under subsection 36(3) of the Consumer Packaging and Labelling Regulations before the day on which these Regulations come into force and in respect of which no decision has been made before that day is deemed to be a request for an exemption under subsection 174(1) of these Regulations.
Foreign systems deemed to be recognized
377 (1) The following systems are deemed to be recognized under Part 7:
- (a) any inspection system of a foreign state for meat products that was, immediately before the day on which these Regulations come into force, approved for the purposes of the Meat Inspection Act;
- (b) any inspection system of a foreign state for live or raw shellfish if that foreign state was, immediately before the day on which these Regulations come into force, authorized in respect of the import of those shellfish into Canada for the purposes of the Fish Inspection Act; and
- (c) any systems of manufacturing, processing, treating, preserving, handling, testing, grading, coding, slaughtering, storing, packaging and labelling that are used in an establishment in a foreign state in relation to meat products if, immediately before the day on which these Regulations come into force, both the establishment and the inspection system of the foreign state for those meat products were approved for the purposes of the Meat Inspection Act.
Suspension and cancellation
(2) For greater certainty, the recognition of any system referred to in subsection (1) may be suspended or cancelled in accordance with Part 7.
PART 16
Consequential Amendments, Repeals and Coming into Force
Consequential Amendments
Canadian Dairy Commission Act
EEC Aged Cheddar Cheese Export Regulations
378 The definition aged cheddar cheese in section 2 of the EEC Aged Cheddar Cheese Export Regulations footnote 1 is replaced by the following:
aged cheddar cheese means Canadian-produced cheddar cheese that is graded Canada 1 under the Safe Food for Canadians Regulations and aged for a period of not less than nine months; (fromage cheddar fort)
Consumer Packaging and Labelling Act
Consumer Packaging and Labelling Regulations
379 The definition wine in subsection 2(1) of the Consumer Packaging and Labelling Regulations footnote 2 is repealed.
380 Section 4 of the Regulations and the heading before it are replaced by the following:
Exemptions from Sections 4, 5, 6 and 10 of the Act
4 Prepackaged products that are subject to regulations respecting packaging, labelling and marking under the Feeds Act, Fertilizers Act, Seeds Act or Pest Control Products Act are exempt from sections 4, 5, 6 and 10 of the Act.
381 (1) Paragraph 5(1)(a) of the Regulations is repealed.
(2) Paragraph 5(2)(a) of the Regulations is repealed.
(3) Paragraph 5(3)(a) of the Regulations is repealed.
382 The definition specialty product in subsection 6(1) of the Regulations is replaced by the following:
specialty product means a prepackaged product that is an imported product
- (a) that is not widely used by the population as a whole in Canada; and
- (b) for which there is no readily available substitute that is manufactured, processed, produced or packaged in Canada and that is generally accepted as being a comparable substitute; (produit spécial)
383 Subsection 14(5) of the Regulations is repealed.
384 Section 18 of the Regulations and the heading before it are repealed.
385 (1) The definition individually measured commodity in subsection 19(1) of the Regulations is repealed.
(2) Subsections 19(2) to (4) of the Regulations are replaced by the following:
(2) Prepackaged products that are packaged from bulk on a retail premises, other than wallpaper or floor covering, are exempt from paragraph 4(1)(b) of the Act and from section 14 of these Regulations, if the net quantity of the product is clearly shown on the principal display panel of its label in terms of a Canadian unit.
386 Section 22 of the Regulations is replaced by the following:
22 The declaration of net quantity of a prepackaged product that is packed for dispensing in aerosol form shall show the net quantity of the product by weight.
387 Subsection 28(2) of the Regulations is replaced by the following:
(2) Despite subsection (1), if a prepackaged product referred to in that subsection consists of less than seven identical products that are packaged separately and those products are labelled to show all of the information required by the Act and these Regulations and that information is clearly visible at the time of sale, no information is required to be shown on the prepackaged product being sold as one unit and the prepackaged product is exempt from sections 4 and 10 of the Act.
388 The heading before section 32 and sections 32 to 34 of the Regulations are repealed.
389 Subsections 36(1) and (2) of the Regulations are replaced by the following:
36 (1) Subject to subsection (3), a prepackaged product consisting of facial tissue, that is manufactured before January 1, 1997, may only be sold in a container whose size corresponds to a net quantity of product
- (a) of a numerical count of less than 50;
- (b) of a numerical count of 50, 60, 100, 120, 150 or 200; or
- (c) of a numerical count of more than 200, if the container is of a size that corresponds to a net quantity of product that is a multiple of 100 units.
(2) Subject to subsection (3), the net quantity of a prepackaged product referred to in subsection (1) shall be shown in terms of numerical count.
390 Section 40 of the Regulations is replaced by the following:
40 If a prepackaged product consisting of liquid is inspected, the net quantity of the prepackaged product shall be determined on the basis of the assumption that the liquid is at a temperature of 20°C (68°F).
Criminal Code
Regulations Excluding Certain Indictable Offences from the Definition of “Designated Offence”
391 (1) Paragraph 1(b) of the Regulations Excluding Certain Indictable Offences from the Definition of “Designated Offence” footnote 3 is repealed.
(2) Paragraph 1(k) of the Regulations is repealed.
(3) Section 1 of the Regulations is amended by striking out “and” at the end of paragraph (m) and by adding the following after that paragraph:
- (m.1) Safe Food for Canadians Act; and
Feeds Act
Feeds Regulations, 1983
392 Subparagraph 19(1)(d.2)(ii) of the Feeds Regulations, 1983 footnote 4 is replaced by the following:
- (ii) a food animal, as defined in section 1 of the Safe Food for Canadians Regulations, that was raised or slaughtered to become an edible meat product;
Food and Drugs Act
Food and Drug Regulations
393 (1) The definition principal display panel in subsection B.01.001(1) of the Food and Drug Regulations footnote 5 is replaced by the following:
principal display panel means, despite the meaning assigned to that term in section A.01.010,
- (a) in the case of a label that is applied to a consumer prepackaged food within the meaning of section 1 of the Safe Food for Canadians Regulations, the principal display panel as described in paragraphs (a) to (c) of the definition of that term in that section,
- (b) in the case of a label that is applied to a prepackaged product other than a consumer prepackaged food subject to the Safe Food for Canadians Regulations, the part of the label that is applied to all or part of any side or surface of the container that is displayed or visible under normal or customary conditions of sale or use and, if the container does not have such a side or surface, the part of the label that is applied to any part of the container except on the bottom, or
- (c) in the case of a label that is applied to a food that is not a prepackaged product, the part of the label that is applied to all or part of the side or surface of the food that is displayed or visible under normal or customary conditions of sale or use; (espace principal)
(2) Paragraph (a) of the definition common name in subsection B.01.001(1) of the Regulations is replaced by the following:
- (a) the name of the food printed in boldface type, but not in italics, in a provision of these Regulations,
394 Section B.01.302 of the Regulations is replaced by the following:
B.01.302 If the label of a multiple-serving prepackaged product indicates that the product contains or, if prepared as directed in or on the package, provides a specified number of servings or portions, that information must be based on the serving of stated size set out in the nutrition facts table for the product.
395 Subsection B.01.402(8) of the Regulations is repealed.
396 Paragraphs B.01.502(2)(b) and (c) of the Regulations are replaced by the following:
- (b) a representation provided for by paragraph 272(1)(f) or section 273 of the Safe Food for Canadians Regulations;
- (c) a representation provided for by column 1 of Table 2 to Volume 7 of the document entitled Canadian Standards of Identity, prepared by the Canadian Food Inspection Agency and published on its website, as amended from time to time;
397 The portion of subsection B.01.513(2) of the Regulations before the table is replaced by the following:
(2) Subsection (1) does not apply to the statement or claim “light” or “léger” when it is used with respect to rum.
398 (1) Paragraphs B.14.018(1)(a) and (b) of the Regulations are replaced by the following:
- (a) in the case of a carcass other than an imported carcass, the grade that was assigned to the carcass under the Safe Food for Canadians Act or a provincial law;
- (b) in the case of an imported beef carcass, the grade that was assigned to the carcass under the Safe Food for Canadians Act or a provincial law or the grade that was assigned to the carcass by a grading authority established under the laws of the country from which the carcass was imported;
(2) Paragraph B.14.018(1)(d) of the Regulations is replaced by the following:
- (d) in the case of a beef carcass, the yield class, if any, that was assigned to the carcass under the Safe Food for Canadians Act.
399 Paragraph B.27.002(2)(a) of the Regulations is replaced by the following:
- (a) the low-acid food is kept under refrigeration and the statement “Keep Refrigerated” and “Garder réfrigéré” is carried on the principal display panel of the label of its container, as well as on the label of its shipping container; or
400 The Regulations are amended by replacing “section 14 of the Consumer Packaging and Labelling Regulations” with “paragraph 229(1)(a) and subsections 229(2) and (3) of the Safe Food for Canadians Regulations” in the following provisions:
- (a) paragraphs B.01.014(a) and (b);
- (b) paragraph B.01.015(1)(a);
- (c) paragraph B.01.016(a);
- (d) paragraph B.01.017(1)(a);
- (e) paragraph B.01.019(a);
- (f) paragraph B.01.020(1)(a);
- (g) paragraphs B.01.022(a) and (b);
- (h) paragraph B.01.023(a);
- (i) subparagraph B.01.035(5)(a)(i); and
- (j) subparagraph B.01.467(2.1)(c)(iii).
Seeds Act
Seeds Regulations
401 (1) The definitions Act and officially recognized laboratory in subsection 2(2) of the Seeds Regulations footnote 6 are replaced by the following:
Act means the Seeds Act; (Loi)
officially recognized laboratory means a seed testing laboratory that is designated by the Minister as an accredited laboratory under section 2.1 of the Act; (laboratoire reconnu officiellement)
(2) Subsections 2(3) and (4) of the Regulations are repealed.
402 Paragraph 13.2(1)(b) of the Regulations is replaced by the following:
- (b) the grader or sampler does not comply with a provision of the Act or these Regulations.
Health of Animals Act
Health of Animals Regulations
403 (1) The definition registered processed egg station in section 2 of the Health of Animals Regulations footnote 7 is repealed.
(2) Paragraph (c) of the definition country of origin in section 2 of the Regulations is replaced by the following:
- (c) with respect to an animal product or animal by-product — other than non-fertilized ova, semen and meat — that has undergone processing that would prevent the introduction of any reportable disease, any disease referred to in Schedule VII and any serious epizootic disease to which the species from which the product or by-product was derived is susceptible and that can be transmitted by the product or by-product, the country in which the product or by-product underwent that processing; (pays d’origine)
(3) Section 2 of the Regulations is amended by adding the following in alphabetical order:
meat means the edible part of a carcass that is the muscle associated with the skeleton, tongue, diaphragm, heart, gizzard or mammalian oesophagus, with or without accompanying and overlying fat, together with those parts of the bones, skin, sinews, nerves, blood vessels and other tissues that normally accompany the muscle and are not ordinarily removed in dressing a carcass, but does not include the muscle associated with the lips, snout, scalp or ears; (viande)
404 Paragraph 5(3)(a) of the Regulations is replaced by the following:
- (a) removed to and destroyed at an establishment where food animals are slaughtered by the holder of a licence that is issued under paragraph 20(1)(b) of the Safe Food for Canadians Act; or
405 Subsection 34(3) of the Regulations is replaced by the following:
(3) Paragraph (2)(a) does not apply to eggs imported into Canada if they are transported under seal of an inspector direct from the place of entry to a processed egg product establishment approved by the Minister.
(4) In subsection (3), processed egg product establishment means an establishment where eggs or processed egg products are processed, treated or preserved by the holder of a licence that is issued under paragraph 20(1)(b) of the Safe Food for Canadians Act.
406 Subsection 175.1(2) of the Regulations is replaced by the following:
(2) Subsection (1) does not apply in respect of an ovine that is transported directly for slaughter either to an establishment where food animals are slaughtered by the holder of a licence that is issued under paragraph 20(1)(b) of the Safe Food for Canadians Act or to an establishment that is registered under an Act of a province that provides for the inspection of ovine carcasses.
Controlled Drugs and Substances Act
Industrial Hemp Regulations
407 The Industrial Hemp Regulations footnote 8 are amended by replacing “under section 14 of the Canada Agricultural Products Act” with “under section 2.1 of the Seeds Act” in the following provisions:
- (a) paragraph 8(1)(k);
- (b) paragraph 13(2)(e); and
- (c) paragraph 31(b).
Customs Tariff
Determination of Country of Origin for the Purposes of Marking Goods (NAFTA Countries) Regulations
408 The note below the heading of Schedule III to the Determination of Country of Origin for the Purposes of Marking Goods (NAFTA Countries) Regulations footnote 9 is replaced by the following:
Note: In accordance with Schedule I to these Regulations, only some goods are required to be marked so as to indicate the country of origin. For the packaging and labelling of food products, the requirements of the Safe Food for Canadians Act continue to apply.
Repeals
Fish Inspection Act
409 The Fish Inspection Regulations footnote 10 are repealed.
Meat Inspection Act
410 The Meat Inspection Regulations, 1990 footnote 11 are repealed.
Canada Agricultural Products Act
411 The following Regulations are repealed:
- (a) the Egg Regulations footnote 12;
- (b) the Fresh Fruit and Vegetable Regulations footnote 13;
- (c) the Honey Regulations footnote 14;
- (d) the Maple Products Regulations footnote 15;
- (e) the Processed Egg Regulations footnote 16;
- (f) the Processed Products Regulations footnote 17;
- (g) the Dairy Products Regulations footnote 18;
- (h) the Licensing and Arbitration Regulations footnote 19;
- (i) the Livestock and Poultry Carcass Grading Regulations footnote 20;
- (j) the Organic Products Regulations, 2009 footnote 21; and
- (k) the Icewine Regulations footnote 22.
Coming into Force
S.C. 2012, c. 24
412 These Regulations come into force on the day on which section 1 of the Safe Food for Canadians Act comes into force, but if they are registered after that day, they come into force on the day on which they are registered.
SCHEDULE 1
(paragraph 11(2)(c) and subparagraph 15(1)(a)(ii))
Exclusions — Foods Used as Grain, Oil, Pulse, Sugar or Beverage
- 1 amaranth
- 2 barley
- 3 buckwheat
- 4 camelina
- 5 canola
- 6 chickpeas
- 7 cocoa beans
- 8 coffee beans
- 9 dry beans
- 10 dry faba beans
- 11 dry peas
- 12 flaxseed
- 13 hemp
- 14 hops
- 15 lentils
- 16 maize (corn)
- 17 millet
- 18 mustard seeds
- 19 oats
- 20 quinoa
- 21 rapeseed
- 22 rice
- 23 rye
- 24 safflower seeds
- 25 sorghum
- 26 soybeans
- 27 sugar beets
- 28 sugar cane
- 29 sunflower seeds
- 30 tea leaves
- 31 triticale
- 32 wheat
- 33 wild rice
SCHEDULE 2
(Sections 179, subsections 180(1), (2) and (4), sections 181 to 183, subsection 184(1), section 185, paragraphs 258(a), 282(1)(a) and 287(1)(a) and subsection 374(3))
Inspection Legends
Figure 1
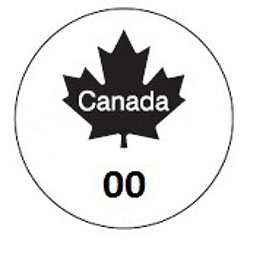
Figure 2

SCHEDULE 3
(Sections 1 and 187, subsection 188(1), sections 189 to 192 and subsection 193(1))
TABLE 1
Consumer Prepackaged Food (Net Quantity by Weight or Volume)
Item |
Column 1 |
Column 2 |
Column 3 |
|---|---|---|---|
1 |
Peanut butter |
250 g |
— |
375 g |
— |
||
500 g |
— |
||
750 g |
— |
||
1 kg |
— |
||
1.5 kg |
— |
||
2 kg |
— |
||
2 |
Wine |
— |
50 mL |
— |
100 mL |
||
— |
200 mL |
||
— |
250 mL |
||
— |
375 mL |
||
— |
500 mL |
||
— |
750 mL |
||
— |
1 L |
||
— |
1.5 L |
||
— |
2 L |
||
— |
3 L |
||
— |
4 L |
||
3 |
Glucose syrup and refined sugar syrup |
— |
125 mL |
— |
250 mL |
||
— |
375 mL |
||
— |
500 mL |
||
— |
750 mL |
||
— |
1 L |
||
— |
1.5 L |
||
— |
2 L |
||
— |
More than 2 L, in increments of 1 L |
TABLE 2
Consumer Prepackaged Food (Net Quantity by Weight)
Item |
Column 1 |
Column 2 |
|---|---|---|
1 |
Honey that is graded in accordance with these Regulations |
150 g or less |
250 g |
||
375 g |
||
500 g |
||
750 g |
||
1 kg |
||
1.5 kg |
||
2 kg |
||
3 kg |
||
5 kg |
||
2 |
Sliced bacon |
100 g or less, in increments of 1 g |
250 g |
||
375 g |
||
500 g |
||
1 kg |
||
3 |
Sliced ready-to-eat meat products and potted meat products |
100 g or less, in increments of 1 g |
125 g |
||
150 g |
||
175 g |
||
200 g |
||
250 g |
||
300 g |
||
375 g |
||
400 g |
||
500 g |
||
600 g |
||
700 g |
||
900 g |
||
1 kg |
||
4 |
Sausages and sausage meat |
100 g or less, in increments of 1 g |
125 g |
||
175 g |
||
225 g |
||
250 g |
||
300 g |
||
375 g |
||
450 g |
||
500 g |
||
600 g |
||
675 g |
||
750 g |
||
900 g |
||
1 kg |
||
5 |
Fresh carrots for which a grade is prescribed by these Regulations |
1.36 kgnote1 or less |
2.27 kgnote2 |
||
4.54 kgnote3 |
||
11.3 kgnote4 |
||
22.7 kgnote5 |
||
6 |
Fresh potatoes for which a grade is prescribed by these Regulations |
1.36 kgnote1 or less |
2.27 kgnote2 |
||
4.54 kgnote3 |
||
9.07 kgnote6 |
||
22.7 kgnote5 |
||
34 kgnote7 |
||
45.4 kgnote8 |
||
7 |
Fresh beets for which a grade is prescribed by these Regulations |
1.36 kgnote1 or less |
2.27 kgnote2 |
||
4.54 kgnote3 |
||
11.3 kgnote4 |
||
22.7 kgnote5 |
||
8 |
Fresh onions for which a grade is prescribed by these Regulations |
1.36 kgnote1 or less |
2.27 kgnote2 |
||
4.54 kgnote3 |
||
11.3 kgnote4 |
||
22.7 kgnote5 |
||
9 |
Fresh parsnips for which a grade is prescribed by these Regulations |
1.36 kgnote1 or less |
4.54 kgnote3 |
||
9.07 kgnote6 |
||
11.3 kgnote4 |
||
22.7 kgnote5 |
||
10 |
Fresh rutabagas for which a grade is prescribed by these Regulations |
1.36 kgnote1 or less |
2.27 kgnote2 |
||
4.54 kgnote3 |
||
11.3 kgnote4 |
||
22.7 kgnote5 |
TABLE 3
Prepackaged Food (Net Quantity by Weight)
Item |
Column 1 |
Column 2 |
|---|---|---|
1 |
Honey that is graded in accordance with these Regulations, other than consumer prepackaged honey that is graded in accordance with these Regulations |
7 kg |
15 kg |
||
30 kg |
||
More than 30 kg, in increments of 1 kg |
||
2 |
Frozen fruits for which a grade is prescribed by these Regulations, with added sugar, syrup, fruit juice or fruit juice from concentrate |
225 g |
425 g |
||
1 kg |
||
1.25 kg |
||
1.5 kg |
||
1.75 kg |
||
2 kg |
||
3 |
Frozen fruits for which a grade is prescribed by these Regulations, dry pack or pie pack, unsweetened, no sugar added |
300 g |
600 g |
||
1 kg |
||
1.25 kg |
||
1.5 kg |
||
1.75 kg |
||
2 kg |
||
4 |
Frozen peas, frozen whole kernel corn and frozen lima beans, for which a grade is prescribed by these Regulations |
350 g or less |
500 g |
||
750 g |
||
1 kg |
||
1.25 kg |
||
1.5 kg |
||
1.75 kg |
||
2 kg |
||
5 |
Frozen spinach for which a grade is prescribed by these Regulations |
300 g or less |
500 g |
||
750 g |
||
1 kg |
||
1.25 kg |
||
1.5 kg |
||
1.75 kg |
||
2 kg |
||
6 |
Frozen mixed vegetables or macédoine, frozen peas and carrots and frozen whole, diced or sliced carrots, for which a grade is prescribed by these Regulations |
300 g or less |
500 g |
||
750 g |
||
1 kg |
||
1.25 kg |
||
1.5 kg |
||
1.75 kg |
||
2 kg |
||
7 |
Frozen special blends or combination mixed vegetables, if the blends or mixed vegetables contain one or more vegetables that are graded in accordance with these Regulations |
300 g or less |
500 g |
||
750 g |
||
1 kg |
||
1.25 kg |
||
1.5 kg |
||
1.75 kg |
||
2 kg |
||
8 |
Other frozen vegetables— including asparagus, broccoli, Brussels sprouts, cauliflower and green and wax beans — for which a grade is prescribed by these Regulations |
300 g or less |
500 g |
||
750 g |
||
1 kg |
||
1.25 kg |
||
1.5 kg |
||
1.75 kg |
||
2 kg |
||
9 |
Frozen cooked squash and frozen diced uncooked squash for which a grade is prescribed by these Regulations |
400 g or less |
750 g |
||
1 kg |
||
1.25 kg |
||
1.5 kg |
||
1.75 kg |
||
2 kg |
||
10 |
Frozen french-fried potatoes for which a grade is prescribed by these Regulations |
225 g or less, in increments of 25 g |
250 g |
||
500 g |
||
1 kg |
||
1.25 kg |
||
1.5 kg |
||
1.75 kg |
||
2 kg |
||
More than 2 kg but not more than 20 kg |
||
11 |
Glace fruits, glace pineapple, cut oranges, lemon and citron peel, cut mixed peel and cut mixed fruit |
100 g |
225 g |
||
450 g |
TABLE 4
Prepackaged Food (Net Quantity by Volume and Container Dimensions)
Item |
Column 1 |
Column 2 |
Column 3 |
Column 4 |
Column 5 |
|---|---|---|---|---|---|
Prepackaged Food |
Net Quantity by Volume |
Container Dimensionsnote1 |
|||
Millilitres/Litres |
Fluid Ounces |
Millimetres |
Inchesnote2 |
||
1 |
Frozen concentrated apple juice or frozen apple juice concentrate for which a grade is prescribed by these Regulations |
177 mL |
6.25 |
54 × 98 |
202 × 314 |
355 mL |
12.5 |
68 × 123 |
211 × 414 |
||
909 mL |
32 |
103 × 142 |
401 × 510 |
||
1.36 L |
48 |
107 × 177 |
404 × 700 |
||
TABLE 5
Food for Which a Grade Is Prescribed by These Regulations if the Container Is a Hermetically Sealed Package (Net Quantity by Volume and Metal Container Dimensions)
Item |
Column 1 |
Column 2 |
Column 3 |
Column 4 |
Column 5 |
|---|---|---|---|---|---|
Net Quantity by Volume |
Metal Container Dimensionsfootnote1 |
||||
Prepackaged Food |
Millilitres/Litres |
Fluid Ounces |
Millimetres |
Inchesfootnote2 |
|
1 |
Fruits packaged with or without water, fruit juice and fruit juice from concentrate, syrup or any combination, heavy pack or solid pack |
142 mL |
5 |
68 x 56 |
211 x 203.5 |
284 mL |
10 |
68 x 101 |
211 x 400 |
||
398 mL |
14 |
76 x 112 |
300 x 407 |
||
540 mL |
19 |
87 x 115 |
307 x 409 |
||
796 mL |
28 |
103 x 119 |
401 x 411 |
||
1.36 L |
48 |
107 x 177 |
404 x 700 |
||
2.84 L |
100 |
157 x 177 |
603 x 700 |
||
2 |
Vegetables other than vegetables for which specific provision is made in this Table |
284 mL |
10 |
68 × 101 |
211 × 400 |
398 mL |
14 |
76 × 112 |
300 × 407 |
||
540 mL |
19 |
87 × 115 |
307 × 409 |
||
796 mL |
28 |
103 × 119 |
401 × 411 |
||
1.36 L |
48 |
107 × 177 |
404 × 700 |
||
2.84 L |
100 |
157 × 177 |
603 × 700 |
||
3 |
Fruit and vegetable juices but not including concentrated apple juice or apple juice concentrate, carbonated juices or juices that are packaged with nitrogen |
200 mL or less |
7 or less |
Any dimensions |
Any dimensions |
250 mL |
8.8 |
Any dimensions |
Any dimensions |
||
284 mL |
10 |
62 × 118 |
207.5 × 410.5 |
||
398 mL |
14 |
76 × 112 |
300 × 407 |
||
500 mL |
17.6 |
Any dimensions |
Any dimensions |
||
540 mL |
19 |
87 × 115 |
307 × 409 |
||
750 mL |
26.4 |
Any dimensions |
Any dimensions |
||
796 mL |
28 |
103 × 119 |
401 × 411 |
||
1 L |
35.2 |
Any dimensions |
Any dimensions |
||
1.36 L |
48 |
107 × 177 |
404 × 700 |
||
1.5 L |
52.8 |
Any dimensions |
Any dimensions |
||
1.82 L |
64 |
Any dimensions |
Any dimensions |
||
2 L |
70.4 |
Any dimensions |
Any dimensions |
||
4 |
Asparagus |
341 mL |
12 |
68 x 115 |
211 x 409 |
540 mL |
19 |
87 x 115 |
307 x 409 |
||
796 mL |
28 |
103 x 119 |
401 x 411 |
||
1.36 L |
48 |
107 x 177 |
404 x 700 |
||
2.84 L |
100 |
157 x 177 |
603 x 700 |
||
5 |
Corn, vacuum pack |
199 mL |
7 |
68 × 82 |
211 × 304 |
341 mL |
12 |
87 × 85 |
307 × 306 |
||
540 mL |
19 |
87 × 115 |
307 × 409 |
||
2.13 L |
75 |
157 × 152 |
603 × 600 |
||
6 |
Mushrooms in brine |
128 mL |
4.5 |
68 × 50 |
211 × 200 |
284 mL |
10 |
68 × 101 |
211 × 400 |
||
398 mL |
14 |
76 × 112 |
300 × 407 |
||
540 mL |
19 |
87 × 115 |
307 × 409 |
||
796 mL |
28 |
103 × 119 |
401 × 411 |
||
1.36 L |
48 |
107 × 177 |
404 × 700 |
||
2.84 L |
100 |
157 × 177 |
603 × 700 |
||
7 |
Tomato paste |
156 mL |
5.5 |
54 × 88 |
202 × 308 |
369 mL |
13 |
76 × 101 |
300 × 400 |
||
796 mL |
28 |
103 × 119 |
401 × 411 |
||
1.36 L |
48 |
107 × 177 |
404 × 700 |
||
2.84 L |
100 |
157 × 177 |
603 × 700 |
||
3.58 L |
126 |
157 × 222 |
603 × 812 |
||
8 |
Tomato pulp, tomato puree and concentrated tomato juice or tomato juice concentrate |
341 mL |
12 |
68 × 115 |
211 × 409 |
540 mL |
19 |
87 × 115 |
307 × 409 |
||
796 mL |
28 |
103 × 119 |
401 × 411 |
||
1.36 L |
48 |
107 × 177 |
404 × 700 |
||
2.84 L |
100 |
157 × 177 |
603 × 700 |
||
3.58 L |
126 |
157 × 222 |
603 × 812 |
||
9 |
Maraschino, creme de menthe and cocktail cherries |
125 mL |
— |
Any dimensions |
Any dimensions |
250 mL |
— |
Any dimensions |
Any dimensions |
||
375 mL |
— |
Any dimensions |
Any dimensions |
||
2 L |
— |
Any dimensions |
Any dimensions |
||
4 L |
— |
Any dimensions |
Any dimensions |
||
10 |
Sweet potatoes, cut |
227 mL |
8 |
68 x 82 |
211 x 304 |
540 mL |
19 |
87 x 115 |
307 x 409 |
||
796 mL |
28 |
103 x 119 |
401 x 411 |
||
1.36 L |
48 |
107 x 177 |
404 x 700 |
||
2.84 L |
100 |
157 x 177 |
603 x 700 |
||
11 |
Sweet potatoes, whole |
597 mL |
21 |
107 x 87 |
404 x 307 |
12 |
Tomato catsup, catsup, tomato ketchup or ketchup |
375 mL |
— |
Any dimensions |
Any dimensions |
540 mL |
19 |
87 × 115 |
307 × 409 |
||
575 mL |
— |
Any dimensions |
Any dimensions |
||
750 mL |
— |
Any dimensions |
Any dimensions |
||
1 L |
— |
Any dimensions |
Any dimensions |
||
1.25 L |
— |
Any dimensions |
Any dimensions |
||
1.5 L |
— |
Any dimensions |
Any dimensions |
||
TABLE 6
Food for Which No Grade Is Prescribed by These Regulations if the Container Is a Hermetically Sealed Package (Net Quantity by Volume and Metal Container Dimensions)
Item |
Column 1 |
Column 2 |
Column 3 |
Column 4 |
Column 5 |
|---|---|---|---|---|---|
Prepackaged Food |
Net Quantity by Volume |
Metal Container Dimensionsnote1 |
|||
Millilitres/Litres |
Fluid Ounces |
Millimetres |
Inchesnote2 |
||
1 |
Beans with pork or beans and pork, beans or vegetarian beans |
128 mL |
4.5 |
68 × 50 |
211 × 200 |
227 mL |
8 |
68 × 82 |
211 × 304 |
||
284 mL |
10 |
68 × 101 |
211 × 400 |
||
398 mL |
14 |
76 × 112 |
300 × 407 |
||
540 mL |
19 |
87 × 115 |
307 × 409 |
||
796 mL |
28 |
103 × 119 |
401 × 411 |
||
1.36 L |
48 |
107 × 177 |
404 × 700 |
||
2.84 L |
100 |
157 × 177 |
603 × 700 |
||
2 |
Infant and junior foods that are processed fruit or vegetable products |
128 mL |
4.5 |
54 × 72 |
202 × 213.5 |
213 mL |
7.5 |
68 × 76 |
211 × 300 |
||
3 |
Vegetable soups, condensed |
284 mL |
10 |
68 × 98 |
211 × 314 |
398 mL |
14 |
76 × 112 |
300 × 407 |
||
540 mL |
19 |
87 × 115 |
307 × 409 |
||
796 mL |
28 |
103 × 119 |
401 × 411 |
||
1.36 L |
48 |
107 × 177 |
404 × 700 |
||
2.84 L |
100 |
157 × 177 |
603 × 700 |
||
4 |
Vegetable soups, ready-to-serve |
227 mL |
8 |
68 × 82 |
211 × 304 |
284 mL |
10 |
68 × 98 |
211 × 314 |
||
398 mL |
14 |
76 × 112 |
300 × 407 |
||
540 mL |
19 |
87 × 115 |
307 × 409 |
||
796 mL |
28 |
103 × 119 |
401 × 411 |
||
1.36 L |
48 |
107 × 177 |
404 × 700 |
||
2.84 L |
100 |
157 × 177 |
603 × 700 |
||
5 |
Spaghetti in tomato sauce |
128 mL |
4.5 |
68 × 50 |
211 × 200 |
227 mL |
8 |
68 × 82 |
211 × 304 |
||
284 mL |
10 |
68 × 101 |
211 × 400 |
||
398 mL |
14 |
76 × 112 |
300 × 407 |
||
540 mL |
19 |
87 × 115 |
307 × 409 |
||
796 mL |
28 |
103 × 119 |
401 × 411 |
||
1.36 L |
48 |
107 × 177 |
404 × 700 |
||
2.84 L |
100 |
157 × 177 |
603 × 700 |
||
6 |
Pineapple, sliced, crushed, tidbits or chunks |
142 mL |
5 |
68 × 56 |
211 × 203.5 |
227 mL |
8 |
87 × 52 |
307 × 201.25 |
||
284 mL |
10 |
68 × 101 |
211 × 400 |
||
398 mL |
14 |
76 × 112 |
300 × 407 |
||
540 mL |
19 |
87 × 115 |
307 × 409 |
||
796 mL |
28 |
103 × 119 |
401 × 411 |
||
1.36 L |
48 |
107 × 177 |
404 × 700 |
||
2.84 L |
100 |
157 × 177 |
603 × 700 |
||
7 |
Grapefruits, oranges and grapefruit and orange sections |
142 mL |
5 |
68 × 56 |
211 × 203.5 |
284 mL |
10 |
68 × 101 |
211 × 400 |
||
398 mL |
14 |
76 × 112 |
300 × 407 |
||
540 mL |
19 |
87 × 115 |
307 × 409 |
||
796 mL |
28 |
103 × 119 |
401 × 411 |
||
1.36 L |
48 |
107 × 177 |
404 × 700 |
||
2.84 L |
100 |
157 × 177 |
603 × 700 |
||
8 |
Fruit juices, including citrus and pineapple juices, but not including lemon, lime, grape, cherry, black currant or raspberry juices, the juices of other berries, carbonated juices or juices that are packaged with nitrogen |
200 mL or less |
7 or less |
Any dimensions |
Any dimensions |
250 mL |
8.8 |
Any dimensions |
Any dimensions |
||
284 mL |
10 |
62 × 118 |
207.5 × 410.5 |
||
398 mL |
14 |
76 × 112 |
300 × 407 |
||
500 mL |
17.6 |
Any dimensions |
Any dimensions |
||
540 mL |
19 |
87 × 115 |
307 × 409 |
||
750 mL |
26.4 |
Any dimensions |
Any dimensions |
||
796 mL |
28 |
103 × 119 |
401 × 411 |
||
1 L |
35.2 |
Any dimensions |
Any dimensions |
||
1.36 L |
48 |
107 × 177 |
404 × 700 |
||
1.5 L |
52.8 |
Any dimensions |
Any dimensions |
||
1.82 L |
64 |
Any dimensions |
Any dimensions |
||
2 L |
70.4 |
Any dimensions |
Any dimensions |
||
9 |
Bean sprouts and vegetables for chop suey |
284 mL |
10 |
68 × 101 |
211 × 400 |
398 mL |
14 |
76 × 112 |
300 × 407 |
||
540 mL |
19 |
87 × 115 |
307 × 409 |
||
796 mL |
28 |
103 × 119 |
401 × 411 |
||
1.36 L |
48 |
107 × 177 |
404 × 700 |
||
2.84 L |
100 |
157 × 177 |
603 × 700 |
||
10 |
Mushrooms, including creamed and stems and pieces in brine |
128 mL |
4.5 |
68 × 50 or 54 × 72 |
211 × 200 or 202 × 213.5 |
284 mL |
10 |
68 × 101 |
211 × 400 |
||
398 mL |
14 |
76 × 112 |
300 × 407 |
||
540 mL |
19 |
87 × 115 |
307 × 409 |
||
796 mL |
28 |
103 × 119 |
401 × 411 |
||
1.36 L |
48 |
107 × 177 |
404 × 700 |
||
2.84 L |
100 |
157 × 177 |
603 × 700 |
||
11 |
Pie fruits, pie fillers and pie fillings |
284 mL |
10 |
68 × 101 |
211 × 400 |
398 mL |
14 |
76 × 112 or 87 × 90 |
300 × 407 or 307 × 309 |
||
540 mL |
19 |
87 × 115 |
307 × 409 |
||
796 mL |
28 |
103 × 119 |
401 × 411 |
||
1.36 L |
48 |
107 × 177 |
404 × 700 |
||
2.84 L |
100 |
157 × 177 |
603 × 700 |
||
12 |
Jams, jellies, marmalades and preserves (conserves), but not including cranberry jelly, jellied cranberries or cranberry sauce |
250 mL or less |
— |
Any dimensions |
Any dimensions |
375 mL |
— |
Any dimensions |
Any dimensions |
||
500 mL |
— |
Any dimensions |
Any dimensions |
||
750 mL |
— |
Any dimensions |
Any dimensions |
||
1 L |
— |
Any dimensions |
Any dimensions |
||
1.5 L |
— |
Any dimensions |
Any dimensions |
||
2 L |
— |
Any dimensions |
Any dimensions |
||
3 L |
— |
Any dimensions |
Any dimensions |
||
4 L |
— |
Any dimensions |
Any dimensions |
||
13 |
Mandarin oranges |
142 mL |
5 |
68 × 56 |
211 × 203.5 |
284 mL |
10 |
75 × 82 |
215 × 304 |
||
2.42 L |
85 |
157 × 155 |
603 × 602 |
||
14 |
Grape juice, concentrated grape juice or grape juice concentrate and grape juice from concentrate, but not including carbonated juices or juices that are packaged with nitrogen |
200 mL or less |
7 or less |
Any dimensions |
Any dimensions |
250 mL |
8.8 |
Any dimensions |
Any dimensions |
||
284 mL |
10 |
62 × 118 |
207.5 × 410.5 |
||
341 mL |
12 |
Any dimensions |
Any dimensions |
||
500 mL |
17.6 |
Any dimensions |
Any dimensions |
||
682 mL |
24 |
Any dimensions |
Any dimensions |
||
750 mL |
26.4 |
Any dimensions |
Any dimensions |
||
1 L |
35.2 |
Any dimensions |
Any dimensions |
||
1.14 L |
40 |
Any dimensions |
Any dimensions |
||
1.36 L |
48 |
107 × 177 |
404 × 700 |
||
1.5 L |
52.8 |
Any dimensions |
Any dimensions |
||
1.82 L |
64 |
Any dimensions |
Any dimensions |
||
2 L |
70.4 |
Any dimensions |
Any dimensions |
||
15 |
Pickles, relishes and chutneys |
125 mL or less |
— |
Any dimensions |
Any dimensions |
250 mL |
— |
Any dimensions |
Any dimensions |
||
375 mL |
— |
Any dimensions |
Any dimensions |
||
500 mL |
— |
Any dimensions |
Any dimensions |
||
750 mL |
— |
Any dimensions |
Any dimensions |
||
1 L |
— |
Any dimensions |
Any dimensions |
||
1.25 L |
— |
Any dimensions |
Any dimensions |
||
1.5 L |
— |
Any dimensions |
Any dimensions |
||
2 L |
— |
Any dimensions |
Any dimensions |
||
2.84 L |
100 |
Any dimensions |
Any dimensions |
||
4 L |
— |
Any dimensions |
Any dimensions |
||
16 |
Green olives, but not including ripe olives, black olives or California ripe olives |
125 mL or less |
— |
Any dimensions |
Any dimensions |
225 mL |
— |
Any dimensions |
Any dimensions |
||
250 mL |
— |
Any dimensions |
Any dimensions |
||
375 mL |
— |
Any dimensions |
Any dimensions |
||
398 mL |
— |
Any dimensions |
Any dimensions |
||
500 mL |
— |
Any dimensions |
Any dimensions |
||
625 mL |
— |
Any dimensions |
Any dimensions |
||
750 mL |
— |
Any dimensions |
Any dimensions |
||
1 L |
— |
Any dimensions |
Any dimensions |
||
1.25 L |
— |
Any dimensions |
Any dimensions |
||
1.5 L |
— |
Any dimensions |
Any dimensions |
||
2 L |
— |
Any dimensions |
Any dimensions |
||
17 |
Sauerkraut with preservative |
284 mL |
10 |
68 × 101 |
211 × 400 |
398 mL |
14 |
76 × 112 or 87 × 90 |
300 × 407 or 307 × 309 |
||
540 mL |
19 |
87 × 115 |
307 × 409 |
||
796 mL |
28 |
103 × 119 |
401 × 411 |
||
909 mL |
32 |
Any dimensions |
Any dimensions |
||
1.36 L |
48 |
107 × 177 |
404 × 700 |
||
2.84 L |
100 |
157 × 177 |
603 × 700 |
||
18 |
Horseradish sauce, prepared horseradish and creamed horseradish |
125 mL or less |
— |
Any dimensions |
Any dimensions |
250 mL |
— |
Any dimensions |
Any dimensions |
||
500 mL |
— |
Any dimensions |
Any dimensions |
||
2 L |
— |
Any dimensions |
Any dimensions |
||
4 L |
— |
Any dimensions |
Any dimensions |
||
TABLE 7
Fresh Vegetables — Volume Capacity of Metric Containers
Item |
Volume Capacity of Metric Containers |
|---|---|
1 |
500 mL |
2 |
1 L |
3 |
2 L |
4 |
4 L |
5 |
6 L |
6 |
13 L |
7 |
18 L |
8 |
36 L |
TABLE 8
Fresh Vegetables — Volume Capacity of Imperial Containers
Item |
Volume Capacity of Imperial Containers |
|---|---|
1 |
1 pint (551 mL) |
2 |
1 quart (1.1 L) |
3 |
2 quarts (2.27 L) |
4 |
4 quarts (4.55 L) |
5 |
6 quarts (6.82 L) |
6 |
11 quarts (12.5 L) |
7 |
16 quarts (18.2 L) |
8 |
32 quarts (36.4 L) |
SCHEDULE 4
(Subsection 199(5) and paragraphs 200(4)(b) and (c))
TABLE 1
Tolerances for Net Quantities Declared in Metric Units of Mass for Consumer Prepackaged Catch-Weight Food
Item |
Column 1 |
Column 2 |
Column 3 |
|---|---|---|---|
1 |
≤ 60 g |
10 |
— |
2 |
> 60 g but ≤ 600 g |
— |
6 |
3 |
> 600 g but ≤ 1 kg |
1 |
— |
4 |
> 1 kg but ≤ 1.5 kg |
— |
10 |
5 |
> 1.5 kg but ≤ 3 kg |
0.66 |
— |
6 |
> 3 kg but ≤ 4 kg |
— |
20 |
7 |
> 4 kg but ≤ 10 kg |
0.5 |
— |
8 |
> 10 kg but ≤ 15 kg |
— |
50 |
9 |
> 15 kg but ≤ 250 kg |
0.33 |
— |
10 |
> 250 kg but ≤ 500 kg |
— |
750 |
11 |
> 500 kg |
0.15 |
— |
TABLE 2
Tolerances for Net Quantities Declared in Canadian Units of Mass or Weight for Consumer Prepackaged Catch-Weight Food
Item |
Column 1 |
Column 2 |
Column 3 |
|---|---|---|---|
1 |
≤ 2 ounces |
10 |
— |
2 |
> 2 ounces but ≤ 20 ounces |
— |
0.2 |
3 |
> 1.25 lb but ≤ 2.2 lb |
1 |
— |
4 |
> 2.2 lb but ≤ 3.3 lb |
— |
0.35 |
5 |
> 3.3 lb but ≤ 6.6 lb |
0.66 |
— |
6 |
> 6.6 lb but ≤ 8.8 lb |
— |
0.71 |
7 |
> 8.8 lb but ≤ 22 lb |
0.5 |
— |
8 |
> 22 lb but ≤ 33 lb |
— |
1.76 |
9 |
> 33 lb but ≤ 550 lb |
0.33 |
— |
10 |
> 550 lb but ≤ 1 100 lb |
— |
26.4 |
11 |
> 1 100 lb |
0.15 |
— |
TABLE 3
Tolerances for Net Quantities Declared in Metric Units of Mass or Volume for Consumer Prepackaged Food Other Than Catch-Weight Food
Item |
Column 1 |
Column 2 |
Column 3 |
|---|---|---|---|
1 |
≤ 50 g or mL |
9 |
— |
2 |
> 50 g or mL but ≤ 100 g or mL |
— |
4.5 |
3 |
> 100 g or mL but ≤ 200 g or mL |
4.5 |
— |
4 |
> 200 g or mL but ≤ 300 g or mL |
— |
9 |
5 |
> 300 g or mL but ≤ 500 g or mL |
3 |
— |
6 |
> 500 g or mL but ≤ 1 kg or L |
— |
15 |
7 |
> 1 kg or L but ≤ 10 kg or L |
1.5 |
— |
8 |
> 10 kg or L but ≤ 15 kg or L |
— |
150 |
9 |
> 15 kg or L |
1 |
— |
TABLE 4
Tolerances for Net Quantities Declared in Canadian Units of Mass or Weight for Consumer Prepackaged Food Other Than Catch-Weight Food
Item |
Column 1 |
Column 2 |
Column 3 |
|---|---|---|---|
1 |
≤ 1.75 ounces |
9 |
— |
2 |
> 1.75 ounces but ≤ 3.5 ounces |
— |
0.16 |
3 |
> 3.5 ounces but ≤ 7 ounces |
4.5 |
— |
4 |
> 7 ounces but ≤ 10.6 ounces |
— |
0.32 |
5 |
> 10.6 ounces but ≤ 17.6 ounces |
3 |
— |
6 |
> 1.1 lb but ≤ 2.2 lb |
— |
0.53 |
7 |
> 2.2 lb but ≤ 22 lb |
1.5 |
— |
8 |
> 22 lb but ≤ 33 lb |
— |
5.28 |
9 |
> 33 lb |
1 |
— |
TABLE 5
Tolerances for Net Quantities Declared in Canadian Units of Volume for Consumer Prepackaged Food Other Than Catch-Weight Food
Item |
Column 1 |
Column 2 |
Column 3 |
|---|---|---|---|
1 |
≤ 1.75 fluid ounces |
9 |
— |
2 |
> 1.75 fluid ounces but |
— |
0.16 |
3 |
> 3.5 fluid ounces but |
4.5 |
— |
4 |
> 7 fluid ounces but |
— |
0.32 |
5 |
> 10.6 fluid ounces but |
3 |
— |
6 |
> 17.6 fluid ounces but |
— |
0.53 |
7 |
> 35.2 fluid ounces but |
1.5 |
— |
8 |
> 2.2 gallons but |
— |
5.28 |
9 |
> 3.3 gallons |
1 |
— |
TABLE 6
Tolerances for Net Quantities of Consumer Prepackaged Food Declared by Numerical Count
Item |
Column 1 |
Column 2 |
|---|---|---|
1 |
< 50 |
0 |
2 |
≥ 50 but ≤ 100 |
1 |
3 |
> 100, with an individual weight of ≤ 14 g or ≤ 0.5 ounce |
0.75% of the declared net quantity, rounded up to the next whole number |
4 |
> 100, with an individual weight of > 14 g or > 0.5 ounce |
0.5% of the declared net quantity, rounded up to the next whole number |
SCHEDULE 5
(Subsection 200(2) and paragraphs 200(4)(a) and (b))
PART 1
Samples
Item |
Column 1 |
Column 2 |
|---|---|---|
1 |
≥ 2 but ≤ 10 |
All the units in the lot |
2 |
≥ 11 but ≤ 128 |
25% of the units in the lot, rounded up to the next whole number, but not less than 10 |
3 |
≥ 129 but ≤ 4 000 |
32 |
4 |
≥ 4 001 but ≤ 8 000 |
64 |
5 |
≥ 8001 but ≤ 12 000 |
96 |
6 |
> 12 000 |
125 |
PART 2
Formula for Determining the Weighted Average Quantity of the Units in a Sample
For the purposes of paragraph 200(4)(a) of these Regulations, the formula for adjusting the sample mean to determine the weighted average quantity of the units in the sample is as follows:
where
![]() is the weighted average quantity of the units in the sample;
is the weighted average quantity of the units in the sample;
![]() is the sample mean calculated as follows:
is the sample mean calculated as follows:
![]()
where
∑x is the sum of the net quantities of all units in the sample;
s is the standard deviation of the sample, calculated as follows:
![]()
where
![]() is the sum of the squared differences between the sample mean and the net quantity of each unit in the sample;
is the sum of the squared differences between the sample mean and the net quantity of each unit in the sample;
t is the value determined in accordance with Part 3 for the selected sample size; and
n is the number of units in the sample.
PART 3
Table for Values of t and (t ÷ √n)
Column 1 |
Column 2 |
Column 3 |
|---|---|---|
2 |
63.657 |
45.01 |
3 |
9.925 |
5.73 |
4 |
5.841 |
2.92 |
5 |
4.604 |
2.06 |
6 |
4.032 |
1.65 |
7 |
3.707 |
1.40 |
8 |
3.499 |
1.24 |
9 |
3.355 |
1.12 |
10 |
3.250 |
1.03 |
11 |
3.169 |
0.955 |
12 |
3.106 |
0.897 |
13 |
3.055 |
0.847 |
14 |
3.012 |
0.805 |
15 |
2.977 |
0.769 |
16 |
2.947 |
0.737 |
17 |
2.921 |
0.708 |
18 |
2.898 |
0.683 |
19 |
2.878 |
0.660 |
20 |
2.861 |
0.640 |
21 |
2.845 |
0.621 |
22 |
2.831 |
0.604 |
23 |
2.819 |
0.588 |
24 |
2.807 |
0.573 |
25 |
2.797 |
0.559 |
26 |
2.787 |
0.547 |
27 |
2.779 |
0.535 |
28 |
2.771 |
0.524 |
29 |
2.763 |
0.513 |
30 |
2.756 |
0.503 |
31 |
2.750 |
0.494 |
32 |
2.746 |
0.485 |
64 |
2.657 |
0.332 |
96 |
2.634 |
0.269 |
125 |
2.615 |
0.234 |
> 32 but |
Value of t to be determined by linear interpolationnote2 |
Value of (t ÷ √n) to be determined in accordance with Part 2 |
PART 4
Minimum Number of Units for the Purposes of Paragraph 200(4)(b)
Item |
Column 1 |
Column 2 |
|---|---|---|
1 |
≥ 2 but ≤ 8 |
1 |
2 |
≥ 9 but ≤ 20 |
2 |
3 |
≥ 21 but ≤ 32 |
3 |
4 |
≥ 33 but ≤ 50 |
4 |
5 |
≥ 51 but ≤ 65 |
5 |
6 |
≥ 66 but ≤ 80 |
6 |
7 |
≥ 81 but ≤ 102 |
7 |
8 |
≥ 103 but ≤ 125 |
8 |
SCHEDULE 6
(Sections 229, and subsection 270(1), paragraphs 312(b), 320(1)(b), 321(c) and 324(a) and section 325)
Minimum Type Size — Principal Display Surface
Item |
Column 1 |
Column 2 |
|---|---|---|
1 |
≤ 32 |
1.6 |
2 |
> 32 but ≤ 258 |
3.2 |
3 |
> 258 but ≤ 645 |
6.4 |
4 |
> 645 but ≤ 2 580 |
9.5 |
5 |
> 2 580 |
12.7 |
SCHEDULE 7
(Section 273)
Identification Names for Food Packaged in Syrup or Fruit Juice
Item |
Column 1 |
Column 2 |
Column 3 |
|---|---|---|---|
1 |
|
|
|
2 |
|
|
|
3 |
|
|
|
SCHEDULE 8
(Subsection 288(1))
Word or Expression on Label of Edible Meat Product
Item |
Column 1 |
Column 2 |
|---|---|---|
1 |
"Baked" or "cuit au four" "Oven Roasted" or "rôti au four" |
Subjected to dry heat without direct contact with a flame for a time sufficient to produce the characteristics of a baked or roasted meat product, such as a brown crust on the surface, rendering of surface fat or caramelization of sugar. The meat product must be ready-to-eat. |
2 |
"Barbecued" or "rôti B.B.Q." |
Cooked with seasoning. The meat product must be ready-to-eat. |
3 |
"Basted" or "arrosé" or "imprégné" "Deep Basted" or "arrosé en profondeur" or "imprégné en profondeur" "Pre-basted" or "préarrosé" or "préimprégné" "Self-basting" or "auto-arrosé" or |
Injected with meat broth that contains at least 15% solid matter, no more than 3% of which is composed of the following ingredients or any combination of them:
|
4 |
"Breaded" or "pané" |
Coated with a combination of batter and bread or cracker crumbs. |
5 |
"Cooked" or "cuit" "Fully Cooked" or "cuit à fond" |
Subjected to heat for a time sufficient to produce the characteristics of a cooked meat product in respect of friability, colour, texture and flavour. The meat product must be ready-to-eat. |
6 |
"Corned" or "traité" |
Cured by adding salt, together with at least 100 ppm of sodium nitrite, potassium nitrite, sodium nitrate or potassium nitrate, or any combination of them, to the meat product. |
7 |
"Dried" or "séché" "Dry"or "sec" "Semi-dry"or "semi-sec" |
Dehydrated. The meat product must be ready-to-eat. |
8 |
"Freeze-dried" or "séché à froid" |
Dehydrated by freeze-drying. |
9 |
"Jellied" or "en gelée" |
Has a gelling agent, as defined in subsection B.01.001(1) of the Food and Drug Regulations, added in an amount exceeding 0.25% of the meat product. |
10 |
"Rolled" or "roulé" |
Boned, rolled and tied. |
11 |
"Semi-boneless"or "semi-désossé" |
At least 45% deboned. |
12 |
"Shankless"or "sans jarret" |
In the case of a foreleg, has the forelimb removed at the elbow joint; in the case of a hind leg, has the hind limb removed at the knee joint. |
13 |
"Smoked" or "fumé" |
Smoked in accordance with the Food and Drug Regulations. |
14 |
"Stuffed" or "farci" "Stuffed with" or "farci de" |
Stuffed with an edible meat product that has been cooked or dehydrated or stuffed with an edible meat product to which has been added any substance other than any edible meat product, or stuffed with one or more of the following ingredients: bread, grains, fruits, nuts, vegetables or similar ingredients. The edible meat product may contain seasoning and animal or vegetable fat. |
15 |
"With Giblets" or "avec abats" or "avec abattis" |
Contains a liver, heart or gizzard or any combination of them from a food animal of the same species. |
16 |
"With Natural Juices" or "avec jus de cuisson" |
Packaged in a package that contains the juices that result from the cooking of the edible meat product. |
SCHEDULE 9
(Clauses 350(1)(c)(i)(B) and (D), paragraph 354(d) and sections 358 and 359)
Product Legend

The product legend is to appear in black with a white background (as illustrated), in black with a transparent background or in colour. If it appears in colour, the background is white or transparent, the outer and inner borders as well as the hills are green (Pantone no. 368), the maple leaf is red (Pantone no. 186) and the lettering is black. / L’estampille comporte un motif noir sur fond blanc (de la façon indiquée), un motif noir sur fond transparent ou est réalisée en couleurs. L’estampille en couleurs comporte un fond blanc ou transparent, des bordures intérieure et extérieure ainsi que des collines vertes (Pantone no 368), une feuille d’érable rouge (Pantone no 186) et des lettres noires.
REGULATORY IMPACT ANALYSIS STATEMENT
(This statement is not part of the Regulations.)
Executive summary
Issues: Recent decades have seen significant changes in the global food environment. Advances in science and technology, the emergence of highly integrated food supply chains and changing consumer preferences require Canada’s federal food regulatory system to keep pace in order to protect the health of Canadians.
The increasingly global marketplace for food commodities has created more opportunities for the introduction and spread of contaminants that may put Canadian food safety at risk. Food-borne illness continues to impose significant health and economic costs on Canadians and recent food safety incidents in Canada have demonstrated where the current federal food regulatory framework must be strengthened. This framework must also keep pace with prevention-focused international food safety standards so that Canadian food exporters have access to foreign markets and remain competitive internationally. Currently, foods preparedfootnote24 in Canada or imported into Canada are not all subject to the same regulatory requirements, and some food safety requirements do not reflect advances in technology, science and food safety best practices.
Description: The Safe Food for Canadians Regulations (the Regulations or SFCR) will strengthen Canada’s reputation as a leader in food safety by establishing consistent, prevention-focused requirements for food that is imported or prepared for export or interprovincial trade, and will also include some requirements applicable to food that is traded intraprovincially. The Regulations will consolidate 13 food commodity-based regulations plus the food-related provisions of the Consumer Packaging and Labelling Regulations (CPLR) into a single regulation under the Safe Food for Canadians Act (SFCA) that is considered to be outcome-based, which means that the regulation is both safe and responsive — they maintain high standards for health and safety while providing flexibility for industry to innovate and compete globally. Some requirements for certain food sectors will be phased in to reflect business size and different levels of industry readiness. Plain-language tools and guidance will be provided to support small businesses that are involved in importing food, or preparing food for export for interprovincial trade, in meeting the requirements.
Cost-benefit statement: The estimated benefits of the Regulations will have an annualized value of approximately $133.1 million. These benefits will be associated with requirements for traceability of food and licensing people as well as the consolidation of food regulations. In comparison, the estimated costs of the requirements will have an annualized value of approximately $127.6 million. These costs will be associated with the use of preventive controls (i.e. food safety requirements) and preventive control plans, the traceability of food, the licensing of people and the Canadian Food Inspection Agency (CFIA) regulatory implementation. The estimated net annualized benefit (i.e. benefits less costs) of these impacts will be approximately $5.4 million.
There will also be significant qualitative benefits, including a reduction in food safety risk for consumers, a more level playing field for Canadian businesses, increased international and domestic regulatory alignment, and sustained market access for Canadian exports. It will also expand the CFIA’s food safety regulatory coverage, bring a more consistent and more effective approach to inspection and oversight for food safety by the CFIA, and enhance Canada’s reputation as a global food safety leader.
“One-for-One” Rule and small business lens: The “One-for-One” Rule will apply. The estimated total administrative cost increase will have an annualized value of approximately $9 million. The small business lens will apply and the CFIA will provide a flexible option for small businesses that are involved in importing food, or preparing food for export or for interprovincial trade. As a result, the estimated total cost savings for these small businesses from the flexible option will have an annualized value of approximately $67 million.
Domestic and international coordination and cooperation: The Regulations will be well aligned with similar modernization efforts among Canada’s key trading partners. In addition, the Regulations will provide a foundation for consistent federal oversight of food that better reflects internationally recognized food safety practices.
Background
Food safety risks have evolved
Canada has one of the best food safety systems in the world but this system must continue to strengthen the oversight of foods that are increasingly at risk of contamination. These high-risk foods include fresh fruits and vegetables and prepared foods that do not fall under the current commodity-based regulations (i.e. foods from what is known as the non-federally registered sector [NFRS]).
As consumers demand more convenient, ready-to-eat products (e.g. bagged salads), the risk of exposure to hazards also increases since these products are intended to be consumed without further cooking. Consumers also increasingly expect foods to be available year-round, which increases demand for imported foods (especially fresh fruits and vegetables) that are often sourced from countries with underdeveloped food safety systems (e.g. from some countries in South America).
The volume of fresh fruits and vegetables and NFRS foods being imported into Canada has approximately doubled, from $11.7 billion in 2006 to $22.8 billion in 2015. With respect to fresh fruits and vegetables, a 43% increase in imports of these products from South America has been observed from 2012–2016.
Over the same period, an increase in food safety issues has been observed from some domestic and foreign sources. From 2011 to 2016, there were 84 recalls related to fresh fruits and vegetables as well as 1 573 recalls related to food from the NFRS. Together, these represented more than 70% of all recalls over this period.
The new risks associated with fresh fruits and vegetables are of particular concern, as this sector is currently not subject to licencing, preventive controls and traceability requirements in Canada. As a result, identification of a food safety hazard is often only possible after illnesses have been reported, rather than through early detection and intervention prior to the entry of food into the retail market. A 2013 study in the Journal of Food Protection demonstrated that from 2001 to 2009, 27 fresh fruit and vegetable-related outbreaks occurred in Canada and resulted in over 1 500 cases of illness.
The import-related aspect of these new risks was illustrated by an incident that resulted in a Canada-wide Salmonella outbreak in 2014. This outbreak was linked to numerous products derived from imported chia seeds and prepared in Canada. It required the recall of 24 products from 9 different manufacturers, with many of these products being from the NFRS. This comprehensive recall was made more complex because of the absence of licensing, preventive control, and traceability requirements for those who imported and prepared these products. The absence of such requirements made it difficult for the CFIA to identify affected food businesses and ensure that the products were removed from the marketplace.
In addition, high-profile food safety incidents have been associated with food from federally registered establishments and have highlighted other areas where the food safety system could be strengthened. For example, a listeriosis outbreak over the summer and fall of 2008 spanned 5 provinces and resulted in 57 human illnesses and 23 deaths. The costs (including medical costs, non-medical costs, productivity losses and federal government costs) associated with this outbreak were estimated to be approximately $242 million. The outbreak was eventually linked to ready-to-eat meat products and a subsequent independent report on the outbreak contained several recommendations. These included suggestions for simplifying and modernizing regulations in accordance with preventive food safety practices and for requiring regulated parties to make the CFIA aware of food safety issues in a timely manner.
A 2012 E. coli outbreak associated with meat products resulted in the largest beef recall in Canadian history, involving the recall and disposal of 12 million pounds of meat products. There were 18 confirmed illnesses and significant economic effects (costs estimated at between $16 million and $27 million) associated with this outbreak. The recommendations that were generated following this incident highlighted, among other things, the need to strengthen requirements for regulated parties to provide adequate documentation in the event of a significant food safety incident.
These events have highlighted the scale and interconnected nature of current production systems, and have also shown that contamination can occur at any stage along the preparation and distribution chains including imported products. These events also underscore the value of preventive approaches (e.g. licensing, preventive controls, and traceability) and the central role that industry has in producing safe food by preventing incidents before they occur rather than dealing with contaminated food once it is on the market.
In light of these challenges, supporting public health and instilling confidence in Canada’s food system remain key priorities for the CFIA’s Food Safety Program. This program aims to mitigate risks to public health associated with diseases and other health hazards related to the food supply system and to manage food safety emergencies and incidents. The program achieves its objectives by promoting food safety awareness and verifying compliance by industry with science-based regulations. The program also delivers initiatives to ensure that consumers receive food safety and nutrition information, and to mitigate unfair market practices that affect consumers and industry. Collaboration with other governments and stakeholders further enhances the Agency’s ability to track, detect and mitigate risks associated with food and the food supply system, including food-borne illness.
Legislative and regulatory context
Five pieces of legislation govern the CFIA’s Food Safety Program: the Canada Agricultural Products Act (CAPA), the Consumer Packaging and Labelling Act (CPLA), the Food and Drugs Act (FDA), the Fish Inspection Act (FIA), and the Meat Inspection Act (MIA).
The regulatory framework underpinning this program is composed of 13 different regulations (plus 2 additional regulations: the Food and Drug Regulations [FDR] and the Consumer Packaging and Labelling Regulations [CPLR]). These include regulations made under the CAPA, the FIA, and the MIA, which cover nine food commodities (i.e. dairy, fish and seafood, fresh fruits and vegetables, honey, maple products, meat, processed eggs, processed [fruit and vegetable] products, and shell eggs).
For each of these food commodities, the CFIA operates food safety, consumer protection, and inspection programs. With respect to certain foods, there are additional requirements found in the Licensing and Arbitration Regulations (LAR), the Livestock and Poultry Carcass Grading Regulations, the Icewine Regulations, and the Organic Products Regulations, 2009.
When it comes fully into force, the SFCA, which received royal assent on November 22, 2012, will repeal and consolidate the CAPA, the FIA, the MIA and the food-related provisions of the CPLA. Once the SFCA is fully in force, all food in Canada within the mandate of the CFIA will be regulated by two federal legislative regimes — the SFCA and the FDA.
International context
Internationally, the CFIA leads the Government of Canada’s implementation of the World Trade Organization’s Agreement on the Application of Sanitary and Phytosanitary Measures, and plays a significant role in the three official international standard-setting bodies to promote science-based international standards. In the case of food, Codex Alimentarius (Codex) standards provide the foundation for robust domestic regulatory systems and contribute to a predictable trade environment, reducing business risks and facilitating market access.
These international approaches to food safety are changing quickly. Codex maintains food standards, guidelines and codes of practice that promote the use of systems-based, preventive approaches to food safety that include Hazard Analysis and Critical Control Point (HACCP)footnote25 principles, Good Manufacturing Practices (GMPs), and Good Agricultural Practices (GAPs). These approaches address safety and quality along the entire food production and distribution continuum by identifying and controlling hazards in order to prevent food safety and quality problems. These systems-based approaches also recognize that those who prepare or import food have the primary responsibility for the safety of that food and must implement preventive programs to identify and control hazards.
Other countries have made significant progress toward adopting the approaches described by Codex as they modernize their own food safety systems. For example, the United States (U.S.) has enacted the Food Safety Modernization Act (FSMA) and associated regulations that grant new and expanded authorities to the U.S. Food and Drug Administration to enhance the safety of the U.S. food supply. Canada and the United States are working together to harmonize regulatory approaches between the two countries where possible, including approaches on food safety. Under the Regulatory Cooperation Council (RCC), the CFIA has been working with its counterparts in the U.S. Department of Agriculture and the U.S. Food and Drug Administration on a number of food safety initiatives (e.g. an arrangement recognizing that the U.S. and Canadian food safety control systems provide a similar level of public health protection), some of which will be furthered by the Regulations.
Issues
Food-borne illness remains a significant public health concern in Canada that causes approximately four million illnesses annually (one in eight Canadians), which results in approximately 238 deaths and 11 600 hospitalizations. A conservative estimate of the annual economic cost to Canadians, the national economy and the health care system is $2.8 billion.footnote26 Recent food safety incidents have demonstrated that changes in consumer preferences and production and distribution systems have produced new food safety challenges. Today, when problems occur, they can affect more products more quickly, and cross into different sectors, into different countries and affect businesses regardless of their size.
Canada’s current federal food regulatory framework has varying requirements and approaches for nine specific food commodities, and has not been regularly updated or streamlined since the CFIA’s creation in 1997. The current framework has no federal requirements for registration or licensing, preventive controls or traceability for food prepared in or imported into Canada other than for some of these nine specific food commodities. As a result, foods prepared in Canada or imported into Canada are not all subject to the same regulatory requirements, and some food safety requirements do not reflect advances in technology, science and food safety best practices.
For example, all food is subject to the FDA, which contains a broad prohibition against the sale of unsafe food; however, this Act does not require licences or registrations for food businesses. It also does not require all food businesses to put in place preventive controls or preventive control plans that are based on HACCP principles. This means that the large majority of foods prepared in Canada or imported are not subject to preventive approaches. Examples of foods in this category include spices, snack foods, baked goods, fats and oils, and infant formula. In practice, this means that preparation and import of some of these foods, which can be as risky as the preparation of foods of federally registered establishments (e.g. meat), are not subject to the same requirements.
These differing approaches among food sectors pose a significant challenge to the CFIA’s goal of managing risks consistently. Differing approaches also mean that businesses involved with multiple food commodities need to meet varying requirements in different regulations, which places an additional burden on these stakeholders.
Further hindering the creation of a level playing field for all Canadian food businesses is the requirement that food businesses preparing food that incorporates more than one food commodity (e.g. a pepperoni pizza) have to comply with multiple sets of applicable requirements (e.g. requirements for grading, labelling, container sizes and weights) in regulations made under the CAPA, the FIA, the MIA and the CPLA. Also, with respect to many exported food, the CFIA does not have the legislative authority to issue certificates that may be required by foreign countries. This may impede market access for some Canadian businesses.
Canada must also keep pace with international food standards and systems, and changes to the food safety systems of Canada’s trading partners so that Canadian food exporters can continue to enjoy access to foreign markets. Taken together, these factors are hindering the creation of a level playing field for all Canadian food business establishments, and have highlighted areas where the current food regulatory framework could be modernized.
Objectives
The key objectives of the Regulations are to
- apply internationally recognized standards for food safety to food that is imported into or prepared in Canada for interprovincial trade or for export. This will better prevent food safety incidents and assist in rapidly removing unsafe food from the market when incidents occur;
- support market access for Canadian exporters by keeping pace with food safety modernization efforts in other countries, such as the United States, who are moving to outcome-based approaches, and strengthening Canada’s reputation for having a world-class food safety system; and
- consolidate 13 food commodity-based regulations plus the food-related provisions of the CPLR to a single set of more outcome-based requirements (i.e. requiring an expected result instead of listing steps to achieve the expected result), where appropriate. This will improve consistency, enable industry innovation and flexibility, and level the playing field across foods and between importers and domestic preparers of food for export or interprovincial trade.
Description
The Regulations will come into force when Section 1 of the SFCA comes into force on the date fixed in the SFCA Order in Council. The Regulations contain 16 parts and will include requirements respecting the following: Trade; Licences; Preventive Controls; Traceability; Commodity-specific Requirements; Recognition of Foreign Systems; Ministerial Exemptions; Inspection Legends; Packaging; Labelling; Grades and Grade Names; Organic Products; and Seizure and Detention. Some of these requirements will be phased in to reflect different levels of industry readiness and the concerns of small businesses that are involved in importing food, or preparing food for export or for interprovincial trade. When it comes fully into force, the SFCA will repeal and consolidate 13 existing regulations plus the food-related provisions of the CPLR. In addition, the Regulations will make consequential amendments to nine regulations under the purviews of the Minister of Agriculture and Agri-Food, Minister of Finance, Minister of Health and Minister of Justice.
The SFCA also provides for authority to incorporate by reference in the Regulations documents that are internally or externally generated as of a particular date or that may change over time. The flexibility to change an internally generated incorporated document will allow the CFIA to make its regulatory framework more responsive to concerns of industry and consumers by responding more promptly to modern science and innovations linked to a regulatory requirement, and which might otherwise require regulatory change. Before making changes to internally generated incorporated documents that may change from time to time, the CFIA will consult with stakeholders in a similar way as consultations for regulatory changes and in accordance with the CFIA’s Incorporation by Reference (IBR) Policyfootnote27 The Regulations incorporate by reference 17 documents, including 11 internally generated documents and 6 externally generated documents as follows:
Internally Generated:
- Ante-mortem Examination and Presentation Procedures for Food Animals
- Biological, Chemical and Physical Standards for Food
- Canadian Grade Compendium
- Canadian Standards of Identity
- Fundamentals of the Post-mortem Examination Program
- Grade Standard Requirements for Fresh Fruits or Vegetables Imported from the United States
- Guidelines for Canadian Drinking Water Quality – Summary Table
- Maximum Quantity Limits for Personal Use Exemption
- Minimum Drained Weights and Average Drained Weights for Processed Fruit or Vegetable Products in a Hermetically Sealed Package
- Preventive Control Plan Requirements for Biological Hazards in Meat Products
- Units of Measurement for the Net Quantity Declaration of Certain Foods
Externally Generated:
- Beef, Bison, and Veal Carcass Grade Requirements
- CAN/CGSB 32.310 standard of the Canadian General Standards Board – Organic Production Systems – General Principles and Management Standards
- CAN/CGSB 32.311 standard of the Canadian General Standards Board – Organic Production Systems – Permitted Substances Lists
- CAN/CGSB 32.312 standard of the Canadian General Standards Board – Organic Aquaculture Standards
- ISO/IEC 17011 standard of the International Organization for Standardization – Conformity assessment – General requirements for accreditation bodies accrediting conformity assessment bodies
- ISO/IEC 17065 standard of the International Organization for Standardization – Conformity assessment – Requirements for bodies certifying products, processes and services
Key food safety elements
The Regulations will establish three key food safety elements:
- (1) Licences: Under the Regulations, licences will be required for food importers, for persons (e.g. food businesses) preparing food for export or for interprovincial trade, with some exceptions (as described in the section “Exceptions and Exemptions”), and for persons slaughtering food animals from which meat products for export or interprovincial trade may be derived. Licence applications will require certain information from the applicant regarding their identity (e.g. business name) and business activities, which will inform risk-based oversight. The licence will be valid for a period of two years for a fee. A licence could be suspended or cancelled. Regulated parties will be able to apply for one or multiple licences. The fee will be set when the CFIA publishes a revised Fees Notice. Before the CFIA introduces this fee, it will be subject to consultation with stakeholders.
- (2) Traceability: The Regulations will reflect the international standard for traceability established by Codex and apply to persons importing, exporting and interprovincially trading food, as well as to other persons holding a licence issued under the SFCA, and to growers and harvesters of fresh fruits or vegetables that are to be exported or traded interprovincially. Documents will be required to be prepared and kept in order to trace food forward to the immediate customer (e.g. a retailer or another food business) and backwards to the immediate supplier (i.e. one step forward, one step back along the supply chain). However, retailers will not be required to trace forward their sales to consumers.
- The Regulations will require that traceability documents be provided, upon the Minister’s request, within 24 hours, or some shorter period, if the information is considered necessary to identify or respond to a risk of injury to human health, or some longer period if the information is not considered necessary for a recall that is or may be ordered. The information will need to be provided in French or in English and, where electronic, in a format that could be imported and manipulated by standard commercial software. The information will need to be accessible in Canada.
- (3) Preventive controls and preventive control plan (PCP): The Regulations will require food subject to the Regulations and activities (e.g. importing, preparing food for export or interprovincial trade) to meet food safety requirements and for those activities to be conducted in a manner that is consistent with internationally recognized agricultural and manufacturing practices (i.e. GAPs, GMPs and HACCP). The Regulations will address the following key preventive control elements:
- sanitation, pest control, and non-food agents;
- conveyances and equipment;
- conditions respecting establishments;
- unloading, loading and storing;
- competency (e.g. for staff);
- hygiene; and
- investigation and notification, complaints and recall.
In addition to these key food safety elements, certain commodity-specific requirements will remain in place where appropriate.
With some exceptions, regulated parties will be required to produce and maintain a written PCP demonstrating how the preventive controls and other requirements (e.g. for packaging and labelling) are met. Where appropriate, regulated parties will have the flexibility to apply the preventive controls and other measures on an outcome-based approach that demonstrates that their activities and food products comply with the Regulations.
The steps related to the preparation of a PCP will be based on HACCP principles and will include, where applicable,
- 1. a description of the biological, chemical, and physical hazards that could contaminate the food, the measures used to prevent or eliminate or reduce to an acceptable level those hazards, and evidence that the measures are effective;
- 2. a description of critical control points (steps at which a control can be applied and that is essential to prevent or eliminate the hazard), their related control measures, and evidence that they are effective;
- 3. a description of the critical limits (i.e. the limit at which a hazard is acceptable without compromising food safety) for each critical control point;
- 4. the procedures for monitoring the critical control points in relation to their critical limits;
- 5. a description of the corrective action procedures for each critical control point;
- 6. a description of the procedures used to verify that the implementation of the PCP meets the requirements of the SFCA and the Regulations;
- 7. in relation to humane treatment requirements:
- a) a description of the measures that will be taken during the handling of food animals to prevent or eliminate a risk of avoidable suffering, injury or death to the animals and during the slaughtering of food animals to prevent or eliminate a risk of avoidable suffering or injury to the animals, and the evidence that shows that each of those measures are effective;
- b) a description of the performance criteria for evaluating the effectiveness of each of those measures;
- c) the procedures for monitoring each of those measures;
- d) the corrective action procedures for each of those measures;
- e) the procedures for verifying that the implementation of the preventive control plan results in compliance with the provisions of the Act and these Regulations; and
- f) the procedures for auditing, on a regular basis, the outcome of the implementation of the preventive control plan;
- 8. documents that demonstrate that the information has been recorded and that the PCP has been implemented with respect to the foregoing;
- 9. supporting documents that show evidence of the information recorded with respect to the foregoing.
Subject to certain exceptions (described in the subsequent section entitled “Exceptions and exemptions”), a written PCP will be required for
- every person who imports food or prepares food to be sent or conveyed from one province to another;
- every person who grows or harvests fresh fruits or vegetables to be exported or to be sent or conveyed from one province to another;
- every person preparing fish products or meat products to be exported; and
- every person exporting food who requires or requests an export certificate from the CFIA.
Exceptions and exemptions
Based on an analysis of the food safety risk, exceptions and exemptions are set out in the Regulations.
There is an exception from the written PCP requirements (licensing, preventive controls, and traceability requirements will still apply) for some regulated parties that generate $100,000 or less in annual gross food sales (i.e. very small and micro-sized businesses). This exception will not apply to regulated parties that slaughter food animals from which meat products for export or inter-provincial trade are derived, or that prepare meat products, dairy products, fish, eggs, processed egg products, or processed fruits and vegetables, or if an export certificate is requested.
A licence would not be required for packaging and labelling of fresh fruits or vegetables in the field by the person who grows or harvests them if they are to be sent or conveyed from one province to another to be subsequently manufactured, processed, treated, preserved or graded by a licence holder.
There are also exceptions from licensing, preventive controls, and written PCP requirements, unless an export certificate is requested, for
- certain alcoholic beverages;
- food additives; and
- some unprocessed foods that will be further prepared (e.g. grains, oilseeds, pulses and other foods such as green coffee beans and hops). These foods must be labelled with the words “For Further Preparation Only,” and may not be prepackaged food for consumers. These foods are listed in Schedule 1 of the Regulations.
The Regulations will also include certain exemptions and exceptions similar to those that exist in current federal regulations, such as food for personal use, food carried on any conveyance that is intended for the crew or passengers, or food for analysis, evaluation, research, or a food exhibition provided that the food is part of a shipment that weighs 100 kg or less or, in the case of eggs, is part of a shipment of five or fewer cases. Food that passes only in transit through Canada will also be excepted, provided the shipment travels in bond.
Importation of non-compliant food (other than meat products) or interprovincial trade of non-compliant food to be subsequently brought into compliance (e.g. through relabelling the food) will be permitted provided that the food is imported by a licence holder, is clearly labelled with “For Further Preparation Only” and is brought into compliance within three months from the day on which it was imported or traded interprovincially, unless a longer time period is granted by the Minister.
Export
Under the SFCA, the Minister may issue export certificates. The Regulations will provide the process by which a regulated party may request an export certificate where one is requested to, for example, fulfill a foreign government requirement, or as in the case of exported meat products to meet the requirements of the SFCR. Under the Regulations, there is an exception to meeting certain requirements of the Regulations with respect to the food being exported where there is a different foreign state requirement on the same matter, and the foreign state requirement is substantiated by documentation to have been met. An exception also applies for certain requirements of the Regulations in respect of the food being exported where there is no foreign state requirement provided the foreign customer specifications are met.
Membership requirements for buyers and sellers of fresh fruits and vegetables
The Licensing and Arbitration Regulations will be repealed, and the Regulations will require that buyers and sellers of fresh fruits and vegetables be members of the Fruit and Vegetable Dispute Resolution Corporation (DRC) to obtain an exception from the prohibition to trade fresh fruits or vegetables. The DRC is a non-profit, membership-based organization serving the produce sector that offers dispute resolution services (e.g. mediation and arbitration) to its members. It should be noted that over 80% of fresh fruit and vegetable buyers and sellers are already members of the DRC. In addition, based on discussions with the fresh fruit and vegetable industry, and their comments during pre-publication, regulations pertaining to the inspection of fresh fruits and vegetables by the CFIA for the resolution of buyer/seller disputes relating to a shipment being damaged or defective will be maintained to allow for CFIA’s Destination Inspection Service to continue.
Meat Products
Changes to requirements for meat products will increase alignment with requirements for other foods to the extent possible, while still considering food safety risks specific to meat products. For example, current mandatory inspection requirements for imported meat products will be removed, and replaced with targeted inspection requirements based on risk. Also, mandatory licensing of operators of meat storage establishments will be removed except for persons who handle and store imported meat products for inspection.
The Regulations will also include exceptions to some existing meat product–specific requirements for meat products that contain a mixture of ready-to-eat meat and other non-meat ingredients (e.g. frozen pepperoni pizza). The Regulations will treat these meat products more similarly to all other prepared foods.
Recognition of foreign systems
The Regulations will prescribe the conditions to be met for the Minister to recognize a foreign system of inspection for meat products and shellfish, and to recognize systems for preparing meat and shellfish in establishments. The Regulations will also provide for circumstances in which ministerial recognition are to be suspended or cancelled including suspending recognition of a foreign meat product establishment where there are a specified number of occurrences of non-compliance for imported meat products from the establishment. When the SFCA fully comes into force (i.e. after the making of the Regulations), systems that are recognized under the MIA or FIA will continue to be recognized under the Regulations.
Ministerial exemptions
The authority for the Minister to exempt food from requirements for the purpose of test-marketing a food that is new or of alleviating shortages will be expanded to all foods. There is also the possibility for an exemption for applying an inspection legend before refrigeration under certain conditions in respect of a carcass or carcass side. Ministerial exemptions may be granted only when they would not result in a risk of injury to human health and, with regards to test-market exemptions, when they would not confuse or mislead the public or disrupt the normal trading patterns of industry or the normal patterns of food pricing.
Inspection legends
Only two figures will be prescribed as inspection legends. A licence holder will be authorized to use an inspection legend under certain conditions and the inspection legend will only apply to meat, fish, and processed egg products.
Container sizes and standard weights
Requirements for standard weight and container sizes under the CAPA, the MIA, the FIA and the CPLA will be included in the Regulations.
Labelling and standards of identity
The Regulations will make some changes to requirements relating to labelling and standards of identity provisions. Changes will group similar provisions together and reduce duplication and differences where possible.
Labelling provisions will be included in the body of the Regulations whereas standards of identity will be incorporated by reference in the Regulations and maintained by the CFIA (in accordance with CFIA’s Incorporation by Reference Policy).
Existing requirements of the CPLA and its regulations apply to prepackaged food sold in Canada, including food sold within a province, and have been included in the Regulations.
Grade requirements
Grade requirements in existing regulations will be consolidated into two documents (noted below) that will be incorporated by reference in the Regulations:
- The Beef, Bison, and Veal Carcass Grade Requirements will be maintained by the Canadian Beef Grading Agency (CBGA) according to conditions outlined in a Memorandum of Understanding between the CBGA and the CFIA.
- The Canadian Grade Compendium will consolidate all other Canadian grade requirements in a single document organized by commodity and maintained by the CFIA.
Organic products
Under the Organic Products Regulations, 2009, only producers of organic products and anyone labelling and packaging organic products were required to be certified. This requirement will be maintained in the SFCR. In addition, the SFCR will give certification bodies the authority to verify compliance of methods and control mechanisms in place as required under the Canadian Organic Standards, as part of the product certification, to allow organic integrity to be maintained along the entire supply chain. In addition, the Regulations will include the organic certification of aquaculture products.
Regulatory and non-regulatory options considered
1. Status quo
The SFCA will not come fully into force and the strengthened authorities provided by the Act will not be put in place. Moreover, the opportunity to streamline and consolidate the existing varying requirements will be lost. Maintaining the status quo will not address new risks facing the Canadian food safety system posed by the continued globalization of the food supply, new products and processing methods, lessons learned from recent food safety incidents, and changing consumer preferences. In addition, the Canadian system will not incorporate new food safety approaches that are internationally accepted and being adopted by Canada’s trading partners which could result in market access issues for Canadian producers.
2. Regulatory option
The regulatory option was chosen, as it is the most effective way to respond to the challenges and opportunities posed to the food safety system as the food industry, global trade in food, food safety risks and food safety risk mitigation approaches all evolve. While this will place additional costs on certain sectors of Canadian industry, the regulatory option is the best means for protecting Canadians from food safety risks while creating a more level playing field for Canadian food businesses.
Benefits and costs
The cost-benefit analysis assessed the potential incremental impacts of the regulatory proposal’s coming into force. The potential impacts (i.e. costs and benefits) represent the incremental differences between the baseline and regulatory scenarios.
The baseline scenario is the situation under the current regulatory framework and what it will look like in the future if the Regulations do not come into force.
The regulatory scenario is the future situation if the Regulations do come into force.
Detailed descriptions of the baseline and regulatory scenario are documented in a cost-benefit analysis report, which is available by request.
Affected stakeholders
Based on the differences between the baseline and regulatory scenarios, the following stakeholders will be affected by the Regulations’ coming into force:
- Food industry businesses
- Preparers of food for interprovincial trade
- Preparers of food for export
- Food importers
- Food exporters
- Interprovincial traders of food
- Fresh fruit and vegetable primary producers
- Organic industry, including certification bodies and conformity verification bodies
- Canadians (i.e. consumers)
- Government
- CFIA
- Health Canada
- Canada Border Services Agency (CBSA)
- Public Health Agency of Canada
- Provincial/territorial governments
Descriptions of the affected stakeholders have been documented in a cost-benefit analysis report, which is available by request.
Identified benefits and costs
This section provides a list and descriptions of some potential benefits and costs that the significant elements of the Regulations may impose on affected stakeholders. These potential impacts represent incremental benefits and costs (i.e. those above and beyond the baseline).
The listing is divided into categories based on benefits/costs that were monetized or benefits that were described qualitatively by the analysis. It should be noted that all significant costs were monetized by the analysis, so no qualitative costs are documented in the Regulatory Impact Analysis Statement (RIAS).
The descriptions of all of the potential benefits and costs have been documented in a cost-benefit analysis report, which is available by request.
Monetized benefits
Review time of CFIA food safety regulations
In the baseline, there are 13 separate sets of CFIA food regulations that need to be reviewed by the food industry, plus the food-related provisions of the CPLR that may need to be reviewed by the food industry (in addition to the FDR). In comparison, in the regulatory scenario, there will only be a single set of CFIA food regulations to be reviewed. As a result, businesses will only need to review one set of regulations instead of potentially multiple sets (e.g. a meat industry business will no longer need to consult the Meat Inspection Regulations, the Livestock and Poultry Carcass Grading Regulations [if applicable], and the CPLR).
Additionally, the regulatory text will be current, and it is expected that the regulatory review time will be reduced, as some of the current CFIA regulations were drafted decades ago and use regulatory text that is outdated and that differs from regulation to regulation. An example of this is in the Fish Inspection Regulations where, unlike in the Meat Inspection Regulations, the definition of exports also includes interprovincial trade.
No establishment registration applications
All food businesses will require a licence, but establishments that are currently required to be registered under the CAPA, FIA, and MIA regulations will no longer need to be registered in the regulatory scenario. Therefore, establishment managers will no longer have to take the time to register. Registration requirements vary across the current regulations, but generally, establishments are required to renew their registrations annually.
Note that the analysis included currently licensed fish or cheese importers in this benefit.
Streamlined/integrated export certification process
Currently, export certification processes differ across the various food commodities. One commonality is that export certification applications are submitted to the CFIA via fax or email. With the current system, applicants receive application status updates by contacting the CFIA and receive their export documents from the CFIA.
The licensing requirements will be supported by a new automated electronic system that will streamline the export certification process. Licence holder information (e.g. licence number, name of licence holder, address[es] of establishment[s]) will be integrated into an online export certification application form. This will provide consistency and efficiencies for both exporters and the CFIA. Additionally, this integration will allow applicants to receive status updates online and print issued certificates online.
More efficient and effective food safety recalls and investigations
As a result of the traceability requirements, recalls and investigations will be conducted in a more efficient and effective manner, which will minimize economic losses for affected businesses. Traceability information will be more readily available and precise. These factors will reduce the duration of recalls/investigations and food waste through improved targeting of affected products, in comparison with the baseline scenario.
CFIA produce licence no longer required
Under the Regulations, fresh fruit and vegetable dealers will not be required to have a CFIA produce licence. Therefore, these dealers will no longer have to take the time to apply for a licence. However, this benefit will be diminished by the fact that these affected stakeholders will have to apply for DRC membership.
Qualitative benefits
Reduced food safety risk
There are approximately four million cases of food-borne illness annually in Canada. This means that one in eight Canadians is affected by food-borne illness every year. Annually, these illnesses result in 11 600 hospitalizations and 238 deaths. A conservative estimate of the annual economic cost this imposes on Canadians, the national economy and the health care system is $2.8 billion.
The Regulations will have stronger food safety requirements than the current requirements under the CAPA, the FIA and the MIA to mitigate the risk of food-borne illness by actively promoting the prevention of food safety incidents. Some examples of requirements that will help achieve this are requirements for preventive controls and written PCPs in food sectors where none were previously required (e.g. currently the non-federally registered and fresh fruit and vegetable sectors).
While the magnitude of the positive impact that these stronger rules will have on the food safety risk for Canadians is uncertain since there was a lack of sufficient information to conduct a proper risk assessment/analysis, it is reasonable to assume that these measures will reduce the risk to some degree, for the following reasons:
- preventive controls and PCP requirements will use a HACCP-based approach to food safety that is systematic and preventive (i.e. catch potential food safety issues before they happen);
- traceability requirements will enable a more rapid response to food safety issues, resulting in less unsafe food reaching consumers;
- licensing requirements will provide the CFIA with a means of communicating with all regulated parties (subject to certain exceptions), which will facilitate an improved emergency response when food safety issues occur.
This reduction in risk will mean that the number of food-borne illnesses across Canada will be reduced when comparing the baseline scenario to the regulatory scenario.
This will in turn reduce the costs to
- Canadians
- reduced number of premature deaths, cases of illness, chronic conditions (i.e. sequelae)
- reduced drug treatment costs, caregiver costs, recovery costs
- the national economy
- reduced productivity loss from worker absenteeism and workers coming in sick and not performing optimally
- reduced number of food safety recalls that businesses need to address
- the health care system
- reduced number of physician visits, hospitalizations, emergency room visits, clinic visits
- reduced drug treatment costs.
Increased international and domestic regulatory alignment
Major trading partners, such as the United States, require PCPs and traceability in their regulatory approaches. Therefore, businesses that develop PCPs (and/or follow the corresponding food safety HACCP-based requirements) and have traceability requirements due to regulatory implementation will benefit from increased alignment with international food safety requirements. Without the proposed PCP, food safety and traceability requirements, Canada will be out of step with its major trading partners that are moving to a preventive control regulatory approach to food safety, putting market access at risk.
Also, international regulatory alignment will increase as a result of the move from differing prescriptive-based regulatory approaches for each regulated food commodity in the baseline to a single outcome-based (where appropriate) regulatory approach for regulated food commodities.
The increased international regulatory alignment has the potential to increase international trade opportunities for the food industry as it will maintain existing market access opportunities for Canadian businesses and support their expansion.
In addition, the outcome-based approach of the Regulations will support provincial/territorial regulatory alignment with federal requirements. With federal regulatory modernization, the opportunity exists to pursue national approaches to food safety, regulatory consistency and greater collaboration between all levels of government. Furthermore, the Regulations will introduce the authority for recognition of inspection and certification systems, which sets the stage for a more fundamental discussion with the provinces and territories on regulatory equivalence.
Outcome-based regulatory approach (where appropriate)
The current regulations under the CAPA, the FIA and the MIA primarily take a prescriptive approach to food safety, which has the potential to limit the way a food business can operate. In comparison, the Regulations will reduce, where appropriate, the current prescriptive food commodity-specific requirements, by moving to a system of requirements that articulates the expected outcomes as they relate to food safety and humane treatment of food animals.
This outcome-based approach will provide businesses with the opportunity for innovation without having to wait for regulatory changes to allow for it, which could lead to reduced compliance costs (e.g. processing costs) over time as businesses find more efficient/effective methods of compliance.
The Regulations will be supported by guidance that provides assistance in meeting the expected outcomes.
More level playing field for food industry
Currently, some food importers and preparers of food for interprovincial trade or for export have to comply with commodity-specific regulatory requirements while others do not. Also, some of these regulatory requirements, such as establishment registration and food safety plans, vary between different commodities.
With the Regulations, the CFIA will move to a single-food regulatory approach. In general, this will mean that there would be a levelling of the competitive playing field for all regulated parties across commodities. Imported food will be held to the same standards and requirements as domestic food.
Enhanced food safety reputation for Canada
The Regulations will implement stronger food safety rules than are currently in place. The strengthened rules will generally apply, with some exceptions, to importers, and preparers of food for export and interprovincial trade and cover all food commodities.
This will enhance Canada’s international reputation as a global food safety leader, which has the potential to lead to increased international trade opportunities for Canadian food businesses by helping to maintain their access to existing markets and support the development of new market access opportunities.
Reduced production costs for processed egg businesses
The processed egg standards of identity will require less egg solid in processing than is currently required. This will reduce production costs for a business that prepares processed egg products.
Additionally, this change will assist in improving the industry’s international trade competitiveness.
Improved CFIA knowledge of food industry
Currently, the CFIA is knowledgeable about food establishments that are registered under the CAPA, FIA and MIA regulations, but has limited to no knowledge of food establishments not covered by these Regulations.
As a result of the licensing requirements, the CFIA will have improved knowledge of the entire food industry (subject to certain exceptions). More specifically, the Agency will know who is importing food, preparing food for interprovincial trade or export, or exporters who require export certification. This will provide the CFIA with a means of communicating with all regulated parties (subject to certain exceptions), which will facilitate an improved emergency response when food safety issues occur. Additionally, the CFIA will be able to more strategically and efficiently focus its food safety efforts based on risk as a result of this improved knowledge.
Moreover, the CFIA will have improved knowledge of the food industry, as the PCP requirements will allow for a consistent single-food inspection approach. This will facilitate a more comprehensive assessment of Canadian food safety, as inspection findings from different food commodity sectors will be more comparable.
Monetized costs
Licence applications
In the regulatory scenario, food importers or preparers of food for interprovincial trade or for export will be required to obtain a licence from the CFIA. Additionally, exporters that need export certification will need a licence. In order to obtain this, a business will have to take the time to apply to the Agency. Licences will be required to be renewed every two years. It should be noted that the licensing fee was not included in this cost, as fee charges are considered to be transfer payments and should not be regarded as economic costs, as per Treasury Board of Canada Secretariat (TBS) cost-benefit analysis guidance. footnote 28
Development and documentation of PCPs
All food importers or preparers of food for interprovincial trade, and preparers of meat and fish products for export, subject to certain exceptions, will be required to develop and document a PCP in the regulatory scenario, unless they already have one in place. The analysis assumed that this will be done at the establishment level. Costs associated with this will include the time needed to complete the plan and potentially hiring external expertise for assistance. It is also expected that the costs associated with PCP development and documentation will increase with the volume and the complexity of the activities being carried out by a food business.
When an export certificate is requested, the exporter and the preparer of the food for export will be required to have a PCP.
Implementation of preventive controls and PCPs (i.e. food safety requirements)
For businesses that do not already have a PCP, once one has been developed and documented, it will have to be implemented. Costs that will be associated with this will include implementing new preventive controls, training and education for employees, equipment changes, verification that preventive controls are working and record keeping. As mentioned above, the magnitude of this cost will increase with the volume and complexity of a food business.
Some stakeholders who choose to be exempted from having a written PCP will still be required to have preventive controls (i.e. food safety requirements) in place. These stakeholders will be required to attest to the CFIA as part of the licence application, that they are eligible for an exception from the written PCP requirements and will meet all the requirements of the Regulations, including having preventive controls in place.
The costs for the implementation of preventive controls and PCPs include the annual costs to perform and document food recall simulation.
Maintenance of PCPs
Where a PCP is required, it will need to be maintained in order to comply with regulatory requirements, and adapt to new or changing practices in the context of the licence holder’s activities. It is assumed by the analysis that this will occur on an annual basis.
Development of traceability systems
In the regulatory scenario, persons importing, exporting and inter-provincially trading food, as well as other persons holding a licence issued under the SFCA, will be required to maintain traceability records. Therefore, traceability systems will need to be developed by these businesses. This will include the costs associated with developing traceability procedures and policies, and tools to be used with the system. The magnitude of this cost will be dependent on the current traceability practices of stakeholders and the scale and size of industry operations being considered.
Implementation of traceability systems
To comply with the regulatory requirements on traceability, businesses will have to develop traceability systems, which would generally be implemented in establishments. Implementation means that regulated parties would have to prepare and keep records on the food commodities supplied to them and the food supplied by them, as well as the locations to which they move foods, and incorporated or source food commodities, before supplying a food to another person.
CFIA regulatory implementation
For the CFIA, regulatory implementation will transform and modernize the Agency’s approach to food safety. However, the CFIA will not require any additional food safety program or inspection funding or resources from current levels, as the Regulations will allow the CFIA to operate more efficiently and redistribute its food safety resources more strategically. That said, there will be some additional CFIA resources required for compliance promotion and industry engagement when the Regulations come into force.
Methodology
This section briefly describes the methodology, data sources and key assumptions used to estimate the monetized (and quantified) benefits and costs. The entire methodology has been documented in a cost-benefit analysis report, which is available by request.
Number of affected food businesses and establishments (i.e. businesses that at a minimum will have to review the Regulations)
The following data sources were used to estimate the number of affected businesses:
- CFIA registered establishment and licence holder lists;
- Statistics Canada’s Business Register data taken from the TBS Regulatory Cost Calculator; and
- CBSA importer databases.
The estimated number of affected businesses is presented in the table below.
Estimated number of affected businesses by the year the business will have to review the Regulations
| Year 1 |
Year 2 |
Year 3 |
Year 4 |
Year 5 |
Year 6 |
Year 7 |
Year 8 |
Year 9 |
Year 10 |
TOTAL |
|---|---|---|---|---|---|---|---|---|---|---|
21,025 |
7,467 |
36,791 |
2,339 |
2,368 |
2,398 |
2,429 |
2,458 |
2,489 |
2,520 |
82,285 |
Note: the table presents the results from June 2018 to May 2028.
The analysis estimated that on average there are approximately 1.25 establishments per business.
The estimated number of affected businesses takes into account the delayed coming into force of six months as well as the phased implementation approach of the Regulations (see table entitled “Overview of Phased Implementation Timelines”)
Current industry practices
The analysis had to account for current industry practices in order to estimate the impacts of moving from the baseline to the regulatory scenario. For example, if a business or establishment is already implementing preventive controls and a PCP, then no costs or benefits will be realized by the business or establishment when the preventive control and PCP requirements will come into force.
Accounting for current industry practices was based on data and information from the following:
- CFIA data on regulated parties that currently implement a HACCP-based food safety plan;
- CanadaGAP® data;
- Conference Board of Canada reports; and
- U.S. Food and Drug Administration (U.S. FDA) cost-benefit analysis.
Note that the analysis only used U.S. data in cases where no Canadian data was available.
Annual growth and turnover in the number of businesses and establishments
CFIA data for registered establishments and licensed importers from 2013 to 2016 was used to estimate the annual growth and turnover in the number of affected businesses and establishments.
Number of small and micro businesses
Data from the Business Register was used to estimate the number of small businesses impacted by the Regulations (i.e. businesses with less than 100 employees). For the estimate of the number of very small and micro businesses (i.e. eligible for exception from the written PCP requirement), where revenue data was not available, it was assumed that these businesses have fewer than five employees.
Model parameters and assumptions
The basic assumptions and parameters that were used in this cost-benefit analysis include the following:
- The analysis covered a 10-year time period from June 2018 to May 2028;
- Discount rate = 7%;
- All monetary values are represented using constant year 2012 prices;
- Wage rate data was from Statistic Canada’s Labour Force Survey (2012) and was obtained via the TBS Regulator Cost Calculator; and
- Wage rates were increased by 25% to account for overhead costs, consistent with the methodology used by TBS.
Monetized benefits and costs
These were the general methodological models used to monetize the most significant impacts.
Benefits — More efficient and effective food safety recalls and investigations
- The costs of recalls and investigations for affected businesses will be reduced because of the traceability requirements.
The following model was used to monetize this impact: CANRC × RSCOPE × NRC × (1 – TCOMP) = impact
- CANRC — the cost of a Canadian food recall was estimated using a food industry article footnote 29 found in a literature review, which was adjusted to the Canadian context.
- The cost of a recall presented costs for a mid-sized processor ranging from $246K to $33.4M in U.S. dollars. These costs were converted to the Canadian context (e.g. size, distribution, productivity of Canadian businesses), which resulted in a range of $139K to $18.8M in Canadian dollars.
- The CFIA classifies recalls into one of three categories based on risk to public health of the unsafe food. It was assumed that a business involved in a
- low-risk recall will incur the low end of the cost range (i.e. $139K);
- high-risk recall will incur the high end of the cost range (i.e. $18.8M); and
- medium-risk recall will incur the mid-point of the cost range (i.e. $9.5M).
- RSCOPE — percent reduction of the cost of a food recall due to traceability was estimated using a report produced for Agriculture and Agri-Food Canada in 2013.
- The report stated that traceability can reduce the scope of a recall by 50% and in some cases by 95%.
- These percentages needed to be lowered to account for the differences in the traceability information that will be required by the Regulations and those presented in the report.
- The analysis assumed that implementing the traceability requirements will reduce the cost of a recall by 25%. That said, there are currently traceability requirements for preparers in the meat and fish sectors. So it was assumed that the cost will be reduced by 12.5% in these sectors.
- NRC — the number of primary recalls (i.e. number of recall incidents, which may have resulted in one recall or multiple recalls) is based on information from the CFIA recall database.
- The estimated number of primary recalls for the base year (2014) was based on a 4-year average (2010−2013):
- Class I (high risk) — 77
- Class II (medium risk) — 85
- Class III (low risk) — 102
- TCOMP — the percentage of businesses that currently have traceability systems in place was based on a Conference Board of Canada report.
- The report stated that 66% of preparers of food for export or inter-provincial trade and 56% of food importers, exporters and inter-provincial traders have a traceability system in place.
- The analysis assumed that 66% of affected businesses have a traceability system in place and this was used as a proxy for the percentage of recalls where a traceability system will be in place (i.e. no benefit from the requirements).
Costs — Development and documentation of PCPs
- These costs represent the time needed to complete the initial plan. The estimated average annualized costs for an impacted business to develop and document a PCP are $214.
- The standard cost model (SCM) was used to monetize this impact (TIME × FREQUENCY × WAGE × POPULATION = impact).
- TIME — estimates based on data/information from a U.S. FDA cost-benefit analysis on the Preventive Controls rule.
- FREQUENCY — this will be a one-time cost for establishments.
- WAGE — assumed a manager will perform this task.
- POPULATION — based on estimates of affected stakeholders.
Costs — Implementation of preventive controls and PCPs
- These costs will include capital costs, training costs, activity costs and record-keeping costs. Depending on the degree to which a company currently has identified its hazards and has implemented preventive controls and a preventive control plan (or any HACCP-based plan), additional compliance costs might stem from costs for purchases (e.g. equipment, installation, containers, etc.) and the time to perform tasks (e.g. training, calibration, monitoring, corrective changes to controls, record-keeping, etc.). A business that currently follows a food safety plan (e.g. HACCP, CanadaGap® or a GFSI benchmarked certification) would have lower costs than a business that has no food safety plan in place.
- The estimated average annualized costs for an impacted domestic or importing business to implement preventive controls and a PCP are $5,852. This cost is an estimated average. There would be some businesses that would have lower costs based on their current food safety practices and the complexity of operations.
- For example, a cookie maker with 2 employees that already has food safety process controls for sanitation and allergens but needs to implement recall procedures, and offer food safety training could have estimated annualized costs of approximately $800. A small business importing pre-packaged chocolates with fewer than five employees that already has recall procedures in place but must implement activities such as process controls for sanitation and allergens, and record-keeping, could have estimated annualized costs of approximately $3,200.
- To reduce the burden and cost on all businesses needing to develop and implement preventive controls and PCPs, the CFIA has developed templates and plain language guides to assist businesses in complying with the requirement.
- The SCM plus additional capital costs were used to monetize this impact.
- TIME — estimates based on data/information from the U.S. FDA cost-benefit analysis on the Preventive Controls rule.
- FREQUENCY — these will be ongoing costs for establishments.
- WAGE — there will be multiple tasks associated with these costs. Depending on the task, it was assumed a manager, supervisor or worker will perform the task.
- POPULATION — based on estimates of affected stakeholders.
- Additional costs — one-time costs (e.g. possible equipment purchases), which were estimated based on data/information from the Preventive Controls rule cost-benefit analysis.
- For those businesses that will not be required to have a written PCP, there will still be costs associated with preventive control requirements. The estimated average annualized costs for an impacted business (i.e. eligible for the exception) to implement preventive controls are $4,622. These costs include the costs associated with attestation, which are estimated to be $12 per business.
- All preventive control implementation costs will be carried by these businesses.
- The costs for these businesses are included in the “Preventive Controls for businesses exempt from PCPs” costs category in the Estimated Annualized Values of the Significant Impacts table in the “Estimated Results” section of the RIAS.
Costs — Maintenance of PCPs
- These costs represent the time required to maintain a PCP. The estimated average annualized costs for an impacted business to maintain a PCP are $361.
The SCM was used to monetize this impact.
- TIME — estimates based on data/information from the U.S. FDA cost-benefit analysis on the Preventive Controls rule.
- FREQUENCY — these will be ongoing costs for establishments starting in the second year when the PCP will be in place.
- WAGE — assumed a manager will perform this task.
- POPULATION — based on estimates of affected stakeholders.
Estimated results
The results for all estimated costs are presented as negative values (e.g. –$1); while results for all estimated benefits are presented as positive values (e.g. $1).
The estimated annualized values of the significant impacts detailed in the Methodological section are presented in the table below.
Estimated annualized values of the significant impacts (in Canadian dollars [CAD], constant year 2012 prices, 2018note 1* present value [PV] base year, 7% discount rate)
| Impact Category — Description |
Annualized Values |
|---|---|
Benefits |
|
Review time of CFIA food safety regulations: Avoided time to review the CAPA, FIA and MIA regulations plus the food-related provisions of the CPLR |
$660,627 |
LICENSING |
|
No establishment registration applications |
$141,840 |
Streamlined / integrated export certification process |
$1,131,418 |
LICENSING TOTAL |
$1,273,258 |
TRACEABILITY |
|
More efficient and effective food safety recalls and investigations |
$132,278,041 |
TRACEABILITY TOTAL |
$132,278,041 |
CFIA fresh fruits and vegetables produce licence no longer required |
$3,505 |
Costs |
|
Review time of CFIA food safety regulations: The SFCR |
-$1,139,190 |
LICENSING |
|
Licence application |
-$149,018 |
LICENSING TOTAL |
-$149,018 |
TRACEABILITY |
|
Development of traceability system |
-$15,371 |
Implementation of traceability system |
-$3,531,127 |
TRACEABILITY TOTAL |
-$3,546,498 |
PREVENTIVE CONTROLS and PCPsnote 1** |
|
Development and documentation of PCP |
-$2,318,963 |
Implementation of preventive controls and PCP |
-$63,294,336 |
Preventive controls for businesses exempt from PCPs |
-$51,951,671 |
Maintenance of PCP |
-$3,905,121 |
PREVENTIVE CONTROLS and PCPs TOTALnote 1** |
-$121,470,102 |
CFIA regulatory implementation |
-$2,468,809 |
The table below provides a summary of all of the potential benefits and costs associated with the regulatory proposal.
Cost-benefit statement (in millions of CAD, constant year 2012 prices, 2018note 2* PV base year, 7% discount rate)
Costs, Benefits and Distribution |
Year 1 |
Year 2 |
Year 5 |
Year 9 |
Year 10 |
Total(PV) |
Annualized Value |
|---|---|---|---|---|---|---|---|
A.1 Quantified impacts ($) — BENEFITS |
|||||||
Food Industry — Small Businesses |
$28.1 |
$58.3 |
$160.5 |
$162.3 |
$162.7 |
$914.7 |
$130.2 |
Food Industry — Medium/Large Businessesnote 2*** |
$0.6 |
$1.3 |
$3.5 |
$3.5 |
$3.5 |
$19.9 |
$2.8 |
CFIA |
$0.0 |
$0.0 |
$0.0 |
$0.0 |
$0.0 |
$0.0 |
$0.0 |
Total Benefitsnote 2** |
$28.8 |
$59.6 |
$164 |
$165.8 |
$166.3 |
$934.7 |
$133.1 |
A.2 Quantified impacts ($) — COSTS |
|||||||
Food Industry — Small Businesses |
-$30.6 |
-$78.4 |
-$147.8 |
-$158.1 |
-$160.9 |
-$873.6 |
-$124.4 |
Food Industry — Medium/Large Businessesnote 2*** |
-$0.2 |
-$0.6 |
-$0.9 |
-$0.9 |
-$0.9 |
-$5.6 |
-$0.8 |
CFIA |
-$3.3 |
-$4.2 |
-$3 |
-$0.0 |
-$0.0 |
-$17.3 |
-$2.5 |
Total Costsnote 2** |
-$34.2 |
-$83.2 |
-$151.7 |
-$159.0 |
-$161.8 |
-$896.5 |
-$127.6 |
NET BENEFITS |
$38.2 |
$5.4 |
|||||
B. Quantified impacts (in non-$) — Positive impacts |
|||||||
Small Businesses — Number of new preventive control plans (PCPs) developed annually |
3,125 |
1,598 |
388 |
422 |
431 |
10,773 |
1,077 |
Medium/Large Businesses — Number of new PCPs developed annuallynote 2*** |
14 |
13 |
1 |
1 |
1 |
43 |
4 |
TOTAL — Number of PCPs developed annually |
3,139 |
1,610 |
389 |
423 |
432 |
10,816 |
1,082 |
C. Qualitative impacts |
|||||||
Consumers |
|||||||
Positive impacts
|
|||||||
Food Industry |
|||||||
Positive impacts
Negative impacts
|
|||||||
Federal Government |
|||||||
Positive impacts
Negative impacts
|
|||||||
Provincial and Territorial Governments |
|||||||
Positive impacts
Negative impacts
|
|||||||
Sensitivity analysis
A sensitivity analysis is the portion of a cost-benefit analysis that attempts to deal with the uncertainty that is inherent in predicting the future. Sensitivity analysis involves changing key parameters and assumptions and assessing how this affects the costs and benefits of the regulatory proposal.
Given the scope of this cost-benefit analysis, there were many uncertain parameters and assumptions that could be varied for the sensitivity analysis. However, the analysis chose to focus on two key parameters and assumptions that affect basically all of the estimated impacts:
- discount rate (3%, 7% and 10%); and
- annual industry growth rates (estimated growth used in the analysis +/− 3 percentage points).
In the case of the food industry growth rate, a higher rate will increase the estimated number of affected businesses, while a lower rate will decrease the number. As for the discount rate, a higher discount rate will place relatively less emphasis on estimated future impacts, while a lower rate will place relatively more emphasis on future impacts.
Note that the medium discount rate and medium food industry growth rates were used to estimate the base results of the cost-benefit analysis (i.e. the results presented in the Cost-Benefit Statement)
Sensitivity analysis — Cost-benefit summary table (CAD, constant year 2012 prices, 2018 PV base year)
| Discount Rate |
Food Industry Growth Rate |
Annualized Benefits |
Annualized Costs |
Net |
|---|---|---|---|---|
Low (3%) |
Medium (annual growth rates) |
$137,241,936 |
-$129,299,400 |
$5,639,357 |
Medium (7%) |
Medium (annual growth rates) |
$133,076,241 |
-$125,280,509 |
$5,326,923 |
High (10%) |
Medium (annual growth rates) |
$129,924,983 |
-$122,263,698 |
$5,077,262 |
Low (3%) |
Low (annual growth rates -3 percentage points) |
$101,587,815 |
-$92,435,516 |
$9,152,299 |
Medium (7%) |
Low (annual growth rates -3 percentage points) |
$99,231,723 |
-$90,241,304 |
$8,990,418 |
High (10%) |
Low (annual growth rates -3 percentage points) |
$97,396,236 |
-$88,550,733 |
$8,845,504 |
Low (3%) |
High (annual growth rates +3 percentage points) |
$184,813,072 |
-$179,810,779 |
$5,002,292 |
Medium (7%) |
High (annual growth rates +3 percentage points) |
$177,926,658 |
-$173,053,774 |
$4,872,884 |
High (10%) |
High (annual growth rates +3 percentage points) |
$172,815,847 |
-$168,062,304 |
$4,753,543 |
The results of the sensitivity analysis suggest that the potential impact of the Regulations will be dependent on industry growth as the net impacts are lower in a low-growth scenario and higher in the medium- and high-growth scenarios.
Additionally, the sensitivity analysis examined the impact on the estimated results based on varying an assumption used for the benefit of more efficient and effective food safety recalls and investigations because of traceability (see table below). The assumption is that affected recalls will be evenly distributed among the risk classifications (i.e. low, medium, high). This assumption was made as food can become unsafe at any point along the food chain and the CFIA does not have information to indicate that the impacts on recalls will vary based on the different risk classifications.
For the sensitivity analysis, the assumption was varied where only low-risk recalls or high-risk recalls will be affected (see table below).
Sensitivity analysis — More efficient and effective food safety recalls and investigations (in millions of CAD, constant year 2012 prices, 2018 PV base year, 7% discount rate)
| Distribution of Impact on Recallsby Risk Classification |
|||
|---|---|---|---|
| Even Distribution |
Only Impacts Low-Risk Recalls |
Only Impacts High-Risk (and Some Medium-Risk) Recalls |
|
Net Annualized Value |
$5.4 |
-$124.7 |
$116.9 |
This analysis shows how the estimated results are dependent on the assumed distribution. However, it should be noted that it is highly unlikely that all of the affected recalls will be either entirely high- or low-risk. This contributed to the rationale used by the analysis in choosing an even distribution.
Distributional analysis
In addition to the distributional impacts on small business presented in the Cost-Benefit Statement table, the analysis also examined the distribution of costs across the currently registered, fresh fruits and vegetables, and non-federally registered sectors. The annualized costs of the Regulations are estimated to be distributed across these sectors as follows: federally registered sector — 22%, fresh fruits and vegetables sector — 32%, and non-federally registered sector — 46%.
The CFIA has raised the threshold for the exception from the written PCP requirement from the original proposed $30,000 to $100,000. This change will result in an estimated annualized cost savings of approximately $9.8 million for small businesses.
The provincial/territorial distribution of establishments for food manufacturing and fresh fruit and vegetable producers is as follows: Alberta — 7%, British Columbia — 21%, Manitoba — 3%, New Brunswick — 4%, Newfoundland and Labrador — 2%, Nova Scotia — 4%, Ontario — 31%, Prince Edward Island — 3%, Quebec — 22%, Saskatchewan — 3%, and Northwest Territories, Nunavut and Yukon — 0.1%.
Conclusions
By focusing on the significant impacts of the regulatory proposal, the cost-benefit analysis estimated the annualized value of the costs and benefits will be approximately -$127.6 million and $133.1 million, respectively. In addition to the significant impacts that the analysis monetized, there will be numerous qualitative impacts including, but not limited to
- a reduction in food safety risk for consumers by putting in place requirements that will support the prevention of food safety incidents before they occur;
- increased international and domestic regulatory alignment that will support the maintenance and expansion of market access for Canadian exports;
- an outcome-based regulatory approach (where appropriate) that results in a more level playing field for food industry businesses that provides them with opportunities for growth and innovation;
- a consistent and more effective food safety approach to inspection and oversight by the CFIA;
- the CFIA’s food safety regulatory coverage expanded to include all food commodities; and
- an enhanced reputation for Canada as a global food safety leader.
The estimated monetized net benefit (i.e. benefits less costs) of the Regulations will have an annualized value of approximately $5.4 million. However, this is a conservative estimate, as the principal benefit of a reduced food safety risk for Canadians was not included as a monetized benefit since there was a lack of sufficient information needed to quantify it. Nevertheless, it will be reasonable to assume that the stronger food safety rules will reduce this risk for Canadians to some degree supporting the prevention of food safety incidents before they occur and supporting more efficient and effective responses when food safety incidents do occur. This change in risk will reduce occurrences of food-borne illness, thereby reducing costs to Canadians, the national economy and the health care system. For example, if the Regulations reduced the occurrences of food-borne illness by
- 1%, the estimated annualized net benefit will increase to approximately $ 33.3 million;
- 5%, the estimated annualized net benefit will increase to approximately $ 144.6 million; and
- 10%, the estimated annualized net benefit will increase to approximately $ 283.8 million.
In addition to an expected reduction in the occurrences of food-borne illness, the stronger food safety rules will increase the confidence Canadians have in the safety of domestic and imported food. Also, consumers will start to see an increase in the compliance of imported food labels with Canadian requirements (e.g. bilingual labels). Finally, regulatory organic requirements will be extended to aquaculture, which will increase consumer confidence in organic aquaculture products and potentially increase consumer access to these products through expanded equivalency arrangements for imports.
The significant changes resulting from regulatory implementation will result in significant benefits for affected businesses. The estimated annualized value of these benefits is $133.1 million. The main driver of the benefits is that the traceability requirements will enable food recalls and investigations to be conducted in a more efficient and effective manner, which will minimize economic loss for affected businesses.
While the benefits for affected businesses will be significant with regulatory implementation, businesses will also carry significant costs from the above-mentioned changes. The estimated annualized value of these costs will be approximately -$125.2 million, which represents less than 2% of the $9 billion in net revenues realized in the Canadian food manufacturing subsector.footnote 30 As with any new additional business cost, there is the potential for the business to attempt to “pass along” the cost to buyers (e.g. consumers). For the food industry that will be impacted by the Regulations, it is a competitive industry where a significant portion of businesses are already compliant with the requirements. Furthermore, because Canada is a small open economy in the global market, imported products coming from the United States (where businesses are already meeting the equivalent requirements such as the preventive controls) further intensify competition in the Canadian market. As a result, it is probable that impacted businesses in Canada will rather absorb the additional costs to at least maintain their current market share. These factors will mitigate the potential impact that the business costs will have on consumer prices while increasing the likelihood that a business will have to absorb the majority of the costs.
The main drivers of the costs are the requirements for affected businesses to have a PCP and follow preventive control requirements. The main benefit for businesses that develop PCPs and follow preventive control requirements will be a reduction in the food safety risk of their product, which will contribute to potential purchasers of their food having increased confidence in its safety. Additionally, these businesses will directly benefit from having increased knowledge of their processes and production and increased alignment with international food safety requirements. This will help to maintain existing market access for Canadian businesses and support the development of new market access opportunities. Also, without the broadened application of preventive control and PCP requirements, Canada will be out of step with our major trading partners who are moving to a preventive control regulatory approach to food safety, which will put market access at risk.
Medium/large businesses will be less affected by the Regulations since most are already in compliance with the requirements. Small businesses will be affected to a greater degree by the Regulations if they are involved in importing food, or preparing food for export or for interprovincial trade.
For the CFIA, regulatory implementation will transform and modernize the Agency’s approach to food safety. However, the CFIA will not require any additional food safety funding or resources from current levels as the Regulations will allow the CFIA to operate more efficiently and redistribute its food safety resources more strategically. Therefore, regulatory implementation will essentially be cost neutral for the CFIA with the exception of compliance promotion and industry engagement, which were estimated to be an annualized cost of approximately –$2.5 million. Note that as a result of CFIA compliance promotion and industry engagement the costs of dealing with importers at the border for CBSA will be negligible.
The entire cost-benefit analysis report is available by request.
“One-for-One” Rule
The “One-for-One” Rule applies and the regulatory proposal will be considered an IN under the Rule, since there will be an overall increase in administrative burden. The additional burden will be primarily associated with the licensing application requirements and the record-keeping associated with the PCP and traceability requirements. However, businesses will benefit from some reduced burden (i.e. administrative relief), which will be primarily a result of the CFIA no longer requiring the registration of certain establishments and the integration of the licensing system with the export certification process in the CFIA’s new automated electronic system.
Since 2010, the CFIA has consulted extensively with businesses and industry associations on potential food safety regulations. In general, businesses have been supportive of the preventive control, PCP, and traceability requirements, which are the requirements that will impose the most administrative burden. However, concerns have been expressed related to the knowledge and capacity of some small businesses in meeting the regulatory requirements given the amount of potential burden.
Based on these concerns and the fact that businesses will carry significant additional administrative costs, the CFIA has designed flexibility into the Regulations (e.g. staggered coming-into-force dates for preventive control, PCP, and traceability requirements) and a comprehensive suite of compliance promotion products for small businesses, to reduce the burden they face, while maintaining food safety standards. There will also be an approximately six month delayed coming into force for the SFCR to facilitate businesses’ readiness and compliance.
The following table presents all of the requirements included in the analysis that will impose administrative burden on or provide administrative relief to businesses:
| Impact Category |
Task Description |
Why is it an "Administrative Burden"? |
Administrative Burden Imposed or Relief Provided |
|---|---|---|---|
Overarching |
Review time of CFIA food safety regulations |
Familiarization with information obligations |
Burden imposed |
Licensing |
Licence applications |
Authorizations |
Burden imposed |
No establishment registration applications |
Authorizations |
Relief provided |
|
Streamlined/integrated export certification process |
Authorizations, filling out forms, compiling data |
Relief provided |
|
Preventive controls and PCPs |
Implementation of preventive controls and PCPs |
Collecting and retaining data |
Burden imposed |
Preventive controls for businesses exempt from PCPs (i.e. costs for attestation and recall simulation documentation) |
Authorizations |
Burden imposed |
|
Traceability |
Implementation of traceability systems |
Collecting and retaining data |
Burden imposed |
Requirements for fresh fruit and vegetable dealers |
CFIA produce licence no longer required (includes DRC membership requirement) |
Authorizations |
Relief provided |
The estimated costs of the administrative burden were based on information gathered from a literature review, cost-benefit analyses from other jurisdictions (e.g. the U.S. Food and Drug Administration), reasonable assumptions and consultation with stakeholders and CFIA subject matter experts.
The following assumptions were used to estimate the administrative burden impacts:
Administrative relief — No establishment registration applications
- Almost all of the estimated benefits associated with no longer requiring establishment registration will provide administrative relief (i.e. the time no longer required to obtain or maintain registration)
SCM variables used to monetize this relief:
- TIME — estimated based on number of data fields required in the application forms (varies based on food commodity and the application type [new, amendment, or renewal]). It was assumed that it will take an individual an average of 15 seconds to fill out a data field. Also, it was assumed that it will take a small business an average of 5 minutes and a medium/large business an average of 15 minutes to obtain and make a copy of any document required for submission (number of documents varies based on food commodity). Finally, the analysis accounted for the fact that application packages can be submitted by mail, fax and email. Also, if required, there can be on-site reviews of food safety plans and construction design layouts
- FREQUENCY — after the initial registration, registration renewal requirements vary by food commodity, but the vast majority require annual renewal (with amendments as required)
- WAGE — it was assumed a manager will perform this task plus a 25% markup for overhead costs
- POPULATION — based on annual estimates of affected establishments used in the cost-benefit analysis
Administrative relief — Streamlined/integrated export certification process
- All estimated benefits associated with a streamlined/integrated export certification process were considered to provide administrative relief (i.e. reduced time to submit information to the CFIA)
SCM variables used to monetize this relief:
- TIME — it was estimated that an exporter will save 10 minutes of their time per application
- FREQUENCY — this is an ongoing task based on applications for export certification
- WAGE — it was assumed a manager will perform this task plus a 25% markup for overhead costs
- POPULATION — The CFIA estimated that there are 165,000 export certificates issued annually. Since the CFIA does not track the number of applications (i.e. successful and unsuccessful), it was assumed that this represents 95% of the total number of annual applications. The annual growth in the number of applications was based on the estimated growth of the food industry used in the cost-benefit analysis
Administrative relief — CFIA fresh fruits and vegetables produce licence no longer required
- Only the estimated benefits associated with the differences in the time to apply, amend or renew a CFIA produce licence versus DRC membership for fresh fruit and vegetable dealers were considered to provide administrative relief (i.e. reduced application time)
The SCM plus other costs were used to monetize this impact:
- TIME — estimated based on number of data fields required in the application forms (varies between CFIA and DRC). It was assumed that it will take an individual an average of 15 seconds to fill out a data field. Also, it was assumed that it will take a small business an average of 5 minutes and a medium/large business an average of 15 minutes to obtain and make a copy of any document required for submission (number of documents varies based on food commodity). Finally, the analysis accounted for the fact that application packages can be submitted by mail, fax and email
- FREQUENCY — after the initial application, CFIA fresh fruits and vegetables licences require annual renewal whereas DRC membership does not need to be renewed
- WAGE — it was assumed a manager will perform this task plus a 25% markup for overhead costs
- POPULATION — based on estimates of affected stakeholders used in the cost-benefit analysis
Administrative burden — Review time of CFIA food safety regulations
- Existing and new businesses will have to take the time to review the Regulations. However, new businesses will not have to take the time to review the current (applicable) CFIA regulations that will be repealed
The SCM was used to monetize this impact:
- TIME — estimated based on consultation with CFIA subject matter experts
- FREQUENCY — this will be a one-time cost for businesses
- WAGE — assumed a manager will perform this task
- POPULATION — based on estimates of affected stakeholders
For the TIME variable, the following table provides CFIA estimates of the time required by a food preparing business (based on employee size) to review a regulation.
Review times for a food preparing business to review a regulation
| Business Size |
Review Time for a Regulation (Hours) for a Food Preparing Business |
||
|---|---|---|---|
| Number of Employees |
Minimum |
Maximum |
Average |
1 to 4 |
3 |
40 |
21.50 |
5 to 99 |
3 |
40 |
21.50 |
100 to 500 |
3 |
40 |
21.50 |
More than 500 |
3 |
40 |
21.50 |
The underlying assumption that explains why the times are the same for every business regardless of size was that reviewing (i.e. reading and understanding) a regulation does not include time to develop compliance strategies (e.g. preventive controls) and therefore will not vary due to operational complexities.
These estimated average regulatory review times represent the starting point used to estimate the times required for all food businesses based on the type of operations. In order to make the estimations, the following assumptions were made:
- The time required to review a regulation will vary based on the operations of the business
- Businesses that do not prepare food will take less time to review since their operations are apt to be less complicated and not all of the regulatory provisions will be applicable
- Compared with the review time for businesses that prepare food, the time will be reduced by
- 75% for importers and exporters
- the assumption being that these businesses do not prepare food at all — i.e. simple operations
- 75% for importers and exporters
- 95% for interprovincial traders
- the assumption being that these businesses only have to comply with minimal requirements
- 0% for fresh fruit and vegetable (FFV) primary producers
- the assumption being that these businesses have complicated operations
- For the Regulations, the CFIA will have interpretive guidance, model systems and plain language examples to help reduce review times
- Additionally, the CFIA will target these documents to specific stakeholder categories (e.g. importer), which will allow stakeholders to only review what will be relevant for them
- These documents will reduce review times by 50%
- For the Regulations, the CFIA has conducted extensive industry consultations on the Regulations, which will help further reduce review times
Based on this, it was assumed that
- all large businesses (more than 500 employees) have been reviewing CFIA regulatory consultation material and are already aware of the majority of the provisions
- as a result their review time will be reduced by 50%
- some medium-sized businesses (100 to 500 employees) have been reviewing CFIA regulatory consultation material and are already aware of the majority of the provisions
- as a result their review time will be reduced by 25%
- a few small businesses (less than 100 employees) have been reviewing CFIA regulatory consultation material and are already aware of the majority of the provisions
- as a result their review time will be reduced by 12.5%
Based on these assumptions, the following table contains the estimated average review times for a single regulation in the baseline and regulatory scenarios for all stakeholder categories:
| Review Time for a Regulation (Hours) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BusinessSize |
BASELINE Regulations |
The SFCR |
||||||||
| Number of Employees |
Preparers forinterprovincial trade or export |
Importers |
Interprovincial traders |
Exporters |
FFV primary producers |
Preparers for interprovincial trade or export |
Importers |
Interprovincial traders |
Exporters |
FFV primary producers |
1 to 4 |
21.50 |
5.38 |
1.08 |
5.38 |
21.50 |
9.41 |
2.35 |
0.47 |
2.35 |
9.41 |
5 to 99 |
21.50 |
5.38 |
1.08 |
5.38 |
21.50 |
9.41 |
2.35 |
0.47 |
2.35 |
9.41 |
100 to 500 |
21.50 |
5.38 |
1.08 |
5.38 |
21.50 |
8.06 |
2.02 |
0.40 |
2.02 |
8.06 |
More than 500 |
21.50 |
5.38 |
1.08 |
5.38 |
21.50 |
5.38 |
1.34 |
0.27 |
1.34 |
5.38 |
It was assumed that the percentage of time required to review the Regulations that will be related to administrative burden requirements will be
- 90% for food retailers
- 90% for interprovincial traders
- 10% for all other impacted stakeholders
For the current regulations, the percentages were assumed to be
- 0% for food retailers
- 0% for interprovincial traders
- 5% for all other impacted stakeholders
Note that for the fish and meat sectors, it was assumed that 10% of the time required to review the current regulations will be related to administrative burden requirements as these regulations have similar requirements as the Regulations (e.g. licensing/registration and record keeping for food safety and traceability). In cases of multi-food businesses that deal with meat and fish, it was assumed that 7.5% of the time will be related to administrative burden.
Administrative burden — Licence applications
- All of the estimated costs associated with licence applications will impose an administrative burden (e.g. the time required to apply for or maintain a licence)
The SCM variables used to monetize this burden:
- TIME — it was assumed that all applications will be submitted electronically via the CFIA website. The time estimated was based on the number of data fields required in the application form (varies based on food commodity and the application type — new, amendment or renewal). It was assumed that it will take an individual an average of 15 seconds to fill out a data field. Also, it was assumed that the form will be “dynamic,” in the sense that some questions (i.e. data fields) will only be presented to the applicant if applicable. For example, questions regarding the types of fish products that an establishment deals with will only be asked to establishments that stated that they deal with fish. Finally, it was assumed that it will take an individual an average of 5 minutes to find the form the first time and 2.5 minutes on subsequent occasions (the underlying assumption being that the CFIA will provide a direct link to the form on its home web page)
- FREQUENCY — after applying for the initial licence, licences will be required to be renewed every two years (with amendments as required)
- WAGE — it was assumed that a manager will perform this task plus a 25% mark-up for overhead costs
- POPULATION — based on estimates of affected businesses used in the cost-benefit analysis
Administrative burden — Implementation of PCPs and preventive control requirements
Only the estimated costs associated with PCP and recall simulation record keeping will impose an administrative burden.
The SCM variables used to monetize this burden:
- TIME — see the table below for the assumptions used for this variable
PCP implementation — Data on administrative burden time by business size (i.e. number of employees)
| Description |
Less than 20 employees |
20 to 99 employees |
100 to 499 employees |
Greater than 499 employees |
|---|---|---|---|---|
Process Controls |
||||
Number of processes per facility |
2 |
2 |
6 |
10 |
Average hours to generate calibration records per process (manager level) |
0.335 |
0.335 |
0.335 |
0.335 |
Number of calibration records per process per year |
24 |
24 |
24 |
24 |
Average hours to document monitoring of process controls per record (working level) |
0.05 |
0.05 |
0.05 |
0.05 |
Monitoring records per process per year |
365 |
365 |
365 |
365 |
Average hours to generate verification instrumentation calibration records per process (manager level) |
0.335 |
0.335 |
0.335 |
0.335 |
Number of calibration records per process per year |
24 |
24 |
24 |
24 |
Allergen Controls — label application review |
||||
Frequency of review per hour per line |
1.5 |
1.5 |
1.5 |
1.5 |
Hours of operation per day |
8 |
16 |
24 |
24 |
Days of operation per year |
357 |
357 |
357 |
357 |
Hours per application record keeping (working level) |
0.013 |
0.013 |
0.013 |
0.013 |
Number of production lines per facility |
3 |
7 |
13 |
18 |
Sanitation Controls — monitoring and verification |
||||
Total hours per year for monitoring record keeping (supervisor level) |
11.125 |
22.375 |
133.875 |
133.875 |
Recall Simulation — document mock recall |
||||
Total hours per year for documentation of the mock recall (manager level) |
0.5 |
0.5 |
0.5 |
0.5 |
- FREQUENCY — these will be ongoing annual costs for establishments
- WAGE — depending on the task, it was assumed that a manager, supervisor or worker will perform the task
- POPULATION — based on estimates of affected establishments used in the cost-benefit analysis
Administrative burden — Preventive controls for businesses exempt from PCPs (i.e. costs for attestation and recall simulation documentation)
The SCM variables used to monetize this burden:
- TIME (attestation) — applicants who seek an exception from a written PCP will go through a modified licence application which includes an additional attestation. The assumed tasks for the process include reading the attestation declaration, selecting the attestation option on the application, collecting sales records and performing sales calculation. The time estimated was based on the number of words in the declaration, the number of data fields required in the application form and the number of data fields required on the calculation table for eligibility for PCP exception. It was assumed that it will take an individual an average one minute to read 300 words and an average of 15 seconds to fill out a data field. Moreover, it was assumed that it will take an individual an average of five minutes to find the sales records or tax records to perform the sales calculation.
- TIME (recall simulation documentation) — see table in section for PCP implementation — Data on administrative burden time by business size (i.e. number of employees)
- FREQUENCY (attestation) — the attestation will be made as part of the licence application process (i.e. when applying for the initial licence and when renewing every two years)
- FREQUENCY (recall simulation documentation) — these will be ongoing annual costs for establishments
- WAGE — it was assumed that a manager will perform these tasks plus a 25% mark-up for overhead costs
- POPULATION — based on estimates of affected businesses used in the cost-benefit analysis that are eligible for the exception from the written PCP requirements
Administrative burden — Implementation of traceability systems
The SCM variables used to monetize this burden:
- TIME — estimated based on data/information from a U.S. FDA cost-benefit analysis on the Establishment and Maintenance of Records Under the Public Health Security and Bioterrorism Preparedness and Response Act of 2002 rule. Reductions to the time estimates were made since the U.S. rule requires the recording of significantly more information
- In cases where a licence holder will have to trace both forward and back, it was assumed that an individual will require approximately 6.5 hours annually for traceability record keeping. In cases where a licence holder will only have to trace either forward or back, it was assumed that half as much time would be required
- FREQUENCY — this will be an ongoing cost for licence holders
- WAGE — assumed an administrative support worker will perform this task
- POPULATION — based on estimates of affected establishments used in the cost-benefit analysis
In the table below, the results for all estimated new administrative burden (i.e. costs) are presented as negative values (e.g. -$1), while the results for all estimated new administrative relief (i.e. benefits) are presented as positive values (e.g. $1).
Estimated annualized values of administrative impacts for the “One-for-One” Rule (CAD, constant year 2012 prices, 2012 PV base yearnote 3**, 7% discount rate)
| Impact Category |
Task Description |
Annualized Values |
|---|---|---|
Overarching |
Review time of CFIA food safety regulations |
-$108,150 |
Licensing |
Licence applications |
-$99,297 |
No establishment registration applications |
$93,052 |
|
Streamlined and integrated export certification process |
$753,912 |
|
Preventive controls and PCPs |
Implementation of preventive controls and PCPs |
-$7,284,288 |
Preventive controls for businesses exempt from PCPs (i.e. costs for attestation and recall simulation documentation) |
-$152,889 |
|
Traceability |
Implementation of traceability systems |
-$2,352,939 |
Requirements for fresh fruit and vegetable dealers |
CFIA produce licence no longer required (includes DRC membership requirement) |
$2,322 |
Total annualizednote 3* administrative impact on all businesses |
-$9,148,276 |
|
Estimated number of affected businesses |
82,285 |
|
Average annualized administrative impact per affected business |
-$111 |
|
The estimated total annualized increase in administrative burden to all businesses will be -$9,148,276. This will equate to an average annualized administrative cost per affected business of $111.
Also, when the Regulations come into force the following regulations will be removed:
- Consumer Packaging and Labelling Regulations (food-related provisions only).
- Dairy Products Regulations
- Egg Regulations
- Fish Inspection Regulations
- Fresh Fruit and Vegetable Regulations
- Honey Regulations
- Icewine Regulations
- Licensing and Arbitration Regulations
- Livestock and Poultry Carcass Grading Regulations
- Maple Products Regulations
- Meat Inspection Regulations
- Organic Products Regulations
- Processed Egg Regulations
- Processed Products Regulations
- Rules of the Board of Arbitration (Agriculture and Agri-Food) (SOR/2000-306)
The entire “One-for-One” Rule analysis is available on request.
Small business lens
Since 2010, the CFIA has consulted extensively with small businesses (including a targeted consultation in 2015), associations that represent small businesses and that serve specific ethnic communities, and organizations that represent and assist small businesses on potential food safety regulations. Feedback has been generally supportive of the regulatory framework; however, concerns were expressed related to the knowledge and capacity of some small businesses in meeting the regulatory framework.
Given these concerns and the fact that small businesses who are subject to the Regulations will carry significant additional administrative and compliance costs due to the Regulations, the CFIA worked on the development of the Regulations and the accompanying compliance promotion products to lower some of the costs faced by small businesses while maintaining food safety standards.
The small business lens compares the estimated costs that will be faced by small businesses in an “initial” regulatory option with a “flexible” (i.e. lower cost) regulatory option. This comparison is made in the Regulatory Flexibility Analysis Statement. Estimates of the costs were based on information gathered from a literature review, cost- benefit analyses from other jurisdictions (i.e. U.S. FDA), reasonable assumptions, and consultation with stakeholders and CFIA staff. For the purposes of the small business lens, the Regulations will represent the flexible option.
This flexible option will include (but will not be limited to)
- “model systems” that will provide examples of processes demonstrated to achieve compliance with the regulatory outcome (best practices);
- plain language guidance documents that will further assist with compliance;
- PCP templates available for small businesses to assist with compliance;
- six months of delayed coming into force to facilitate businesses’ readiness and compliance;
- staggered coming-into-force dates for certain requirements for food sectors where no registration requirement for establishments currently exists (e.g. non- federally register sector), which will provide additional time to understand the requirements and delay the costs for compliance; and
- an exception from the PCP requirements for businesses with annual gross food sales of $100,000 or less for businesses in the non-federally registered, fresh fruits and vegetables, maple or honey sectors. However, businesses subject to this exception will still have to adhere to preventive control requirements.
For the small business lens, the initial option for the design of the Regulations would not have any model systems, plain language guidance, PCP templates or exception from PCP requirements. Additionally, there will be a single coming-into-force date for all regulatory provisions.
In the table below, the results for all estimated costs are presented as negative values (e.g. -$1). Note that the small business lens only analyzes costs (i.e. no benefits are included).
Regulatory Flexibility Analysis Statement (in millions of CAD, constant year 2012 prices, 2018 PV base yearnote 4**, 7% discount rate)
| Initial Option |
Flexible Option |
|||
|---|---|---|---|---|
Short description |
No model systems provided to businesses No "plain language" resources provided to businesses No delayed coming into force for all businesses A single coming-into-force date No micro-business exception from PCP requirements No PCP templates available for businesses |
Model systems provided to businesses "Plain language" resources provided to businesses Six-month delayed coming into force for all businesses Staggered coming-into-force dates for certain food sectors Micro-business exception from PCP requirements PCP templates available for businesses |
||
Number of small businesses impactednote 4**** |
83 179 |
80 923 |
||
Annualized Value ($) |
Present value |
Annualized Value ($) |
Present value |
|
Compliance costs |
||||
Review time of CFIA food safety regulationsnote 4*** |
-$838,753 |
-$5,891,053 |
-$313,959 |
-$2,205,114 |
Development of traceability system |
-$16,525 |
-$116,063 |
-$15,011 |
-$105,432 |
Development and documentation of PCP |
-$8,764,144 |
-$61,555,678 |
-$2,297,537 |
-$16,136,939 |
Implementation of preventive controls and PCP |
-$138,652,659 |
-$973,838,260 |
-$51,810,285 |
-$363,893,763 |
Preventive controls for businesses exempted from PCPs |
$0 |
$0 |
-$51,715,977 |
-$363,231,382 |
Maintenance of PCP |
-$9,650,149 |
-$67,778,610 |
-$3,884,125 |
-$27,280,472 |
TOTAL compliance costsnote 4* |
-$157,922,231 |
-$1,109,179,663 |
-$110,036,934 |
-$772,853,379 |
Administrative costs |
||||
Review time of CFIA food safety regulations |
-$282,749 |
-$1,985,909 |
-$160,256 |
-$1,125,574 |
Licence application |
-$164,828 |
-$1,157,682 |
-$141,901 |
-$996,656 |
Implementation of traceability system |
-$4,225,343 |
-$29,677,042 |
-$3,443,235 |
-$24,183,845 |
Implementation of PCP |
-$29,561,552 |
-$207,627,973 |
-$10,836,467 |
-$76,110,809 |
Preventive controls (i.e. attestation and recall simulation documentation) |
NA |
NA |
-$229,445 |
-$1,611,523 |
TOTAL administrative costs |
-$34,234,472 |
-$240,448,607 |
-$14,811,660 |
-$104,030,904 |
Total costs (all small businesses) |
-$192,156,703 |
-$1,349,628,270 |
-$124,848,594 |
-$876,884,283 |
Total cost per impacted small business |
-$2,310 |
-$16,226 |
-$1,543 |
-$10,836 |
Risk considerations |
Having no model systems, plain language tools or PCP templates combined with no delayed coming into force and a single coming-into-force date for all regulatory requirements will make it more difficult for small businesses to comply. This will put food safety at risk. |
Model systems, plain language tools and PCP templates combined with staggeredcoming-into-force dates and 6-month delayed coming into force for regulatory requirements will assist small businesses with compliance. This will reduce food safety risk. Excepted micro-businesses will still have to comply with food safety requirements. Also, the exception will only apply to very small businesses that will typically have less complex food operations (i.e. less risk of food contamination) than small, medium or large businesses. |
||
The flexible option is recommended for the design of the Regulations by the CFIA. It was estimated that this option will reduce the average annualized cost per affected business from approximately $2,310 (i.e. initial option) to $1,543 (i.e. flexible option). This will result in an estimated average annualized savings of $ 767 per affected small business. The total savings for all small businesses will have an annualized value of $67 million.
It is estimated that approximately 11 263 businesses that do not already have a PCP will be eligible for the exception from the PCP requirements.
For small businesses in the food manufacturing sector, the average net profit was +$18,600 in 2014. So the estimated costs per impacted small business will represent about 8.3% profits. That said, in this sector 70% of businesses are profitable versus 30% that are non-profitable. The profitable businesses have an average net profit of $64,000, so the impact of the costs on profit will be less (2.4%). The non-profitable businesses have an average net loss of $85,000 so the additional costs will not significantly increase these losses.
The completed Small Business Lens Checklist is included as an appendix to the RIAS. The entire small business lens analysis is available by request.
Consultation
The CFIA undertook significant engagement with stakeholders as it developed the proposed Regulations. This included hosting two major food safety forums in 2013 and 2014 which were attended by industry, academia, consumer groups, and other stakeholders. In addition, the CFIA held a focused consultation with very small and micro businesses in 2015 which occurred through multiple channels including face-to-face sessions, webinars, and an on-line survey. Also, a version of the draft preliminary regulatory text, as it then read, was released publicly for stakeholder review and comment in April 2015.
The SFCR was prepublished in the Canada Gazette, Part I on January 21, 2017. The CFIA received 1717 submissions from a variety of stakeholders including industry, consumers, provincial governments, and five foreign governments. Of these submissions approximately half were generated by an animal welfare-related write-in campaign. Of the remaining submissions, approximately a quarter were related to the requirements for organic products and a quarter related to other topics.
Following prepublication, the CFIA conducted additional consultations which reached more than 1500 industry, government and consumer stakeholders and over 500 CFIA staff. These events included: 7 value chain round tables, 23 unique industry outreach events, 16 international events, 5 external webinars, 8 internal webinars, 13 sector specific technical sessions and 10 public information sessions in 9 provinces.
Key messages and themes expressed by stakeholders
As was observed in previous consultations in 2013, 2014 and 2015, stakeholders expressed general support for the regulatory approach. In particular, stakeholders supported the proposed use of outcomes-based approaches that are based on science and rooted in international standards. Stakeholders expressed the belief that the Regulations strike an appropriate balance given the diversity of food businesses in Canada, and enable innovation and flexibility, while levelling the playing field between importers and domestic farmers.
Concerns
A number of concerns were highlighted regarding the proposal:
1. Animal Welfare Issues
Some comments submitted by stakeholders indicated that while they appreciated the modifications that the SFCR made to CFIA’s oversight of animal welfare during slaughter and transportation, the submissions requested that the CFIA consider further modifications. These requests included the use of video surveillance, increased training requirements for those handling animals, and restrictions on the use of electric prods.
CFIA Response:
Certain humane treatment provisions were modified based on the feedback received during the prepublication period, which included additional requirements regarding necessary competencies for persons involved in the handling of food animals during any activity including slaughter, new restrictions on the use of electric prods, and new rules for the segregation and isolation of injured or diseased animals. These additional requirements are consistent with requirements of proposed amendments to the Health of Animals Regulations related to humane transport and will contribute to increased animal welfare and thereby food safety within Canada.
2. Requirements for Organic Products
A significant number of stakeholders did not support the mandatory certification requirements for other service providers (such as operators that slaughter, store and/or convey organic products in international and interprovincial trade) in order to enhance the organic integrity along the entire supply chain. It was noted that while this would facilitate exports of these products to some countries, this could negatively affect the competitiveness of domestic businesses.
CFIA Response:
The CFIA removed the requirement for mandatory certification of activities across the supply chain and will retain its current approach of requiring mandatory certification for organic products and packaging and labelling activities only. In order to continue to facilitate exports, the CFIA has included a requirement for certification bodies to verify conformity of the activities with the Canadian Organic Standards as part of the organic product certification. The methods that are used and the control mechanisms that are in place for every organic product must meet the requirements and the general principles of the applicable Canadian Organic Standard.
3. Effects on Small Business:
Some stakeholders noted that the Regulations would be complex and costly to implement. They requested that the phase-in period for the SFCR requirements be extended, or that additional time be granted to small business to understand and comply with the Regulations. In addition, small businesses and farm producers identified the cost of a written preventive control plan as a costly and unnecessary burden that would place small business at a competitive disadvantage. These stakeholders requested that the CFIA raise the $30,000 threshold below which a written preventive control plan is not required. Stakeholders representing larger businesses and industry associations reiterated previous comments asking that any exception threshold be minimized or removed.
CFIA Response:
In order to offer stakeholders with additional time to read and understand the SFCR (and associated guidance) and to implement any new requirements, there will be an approximately six month delayed coming into force of the Regulations after their publication in Canada Gazette, Part II. During this period, all regulated parties will have the opportunity to review and understand the new requirements and apply guidance material that will be incrementally released leading up to the final publication. Certain requirements will continue to be phased in over the next 2.5 years to reflect business size and different levels of industry readiness by sector.
Additionally, the CFIA has raised the threshold for the exception from the written preventive control plan requirement to $100,000. This threshold strikes a balance between stakeholder comments seeking an increase to the threshold and those asking that eligibility for that exception be kept at a minimal level.
4. Technical Comments:
Submissions sought clarification of terms contained within the proposal to better reflect international standards, or to clarify some requirements to better reflect their outcome-based nature. Industry sought these changes to provide additional flexibility in implementing some requirements.
CFIA Response:
The CFIA modified several provisions in light of the comments received. For example, the CFIA modified a requirement in the Preventive Controls section to more closely align with the Codex Alimentarius approach to preventing, eliminating or reducing hazards to an acceptable level. The CFIA also modified a number of Preventive Controls provisions related to facilities, conveyances and equipment to make them more clearly outcome-based and to facilitate their implementation by industry. This included modifying the preventive control relating to “Hand Cleaning and Sanitizing Stations” to accommodate scientifically justified methods of hand cleaning that prevent contamination of food that are not available in certain locations (e.g. the use of hand wipes plus hand sanitizer in fields during harvest of fresh fruit and vegetables where running water may not be available).
5. Scope:
A small number of comments requested that the CFIA expand the scope of the Regulations to explicitly cover all aspects of the food supply chain such as transportation, or to cover new sectors such as retail food service.
CFIA Response:
The CFIA expects that the design of the Preventive Controls and written Preventive Control Plan will address any hazards related to the transportation or receiving of food and ingredients. The regulation of retail food service establishments will be re-evaluated during a future phase of the Regulations.
6. Continuing to Make Implementation Resources Available
Additionally, stakeholders commented that the guidance materials and interactive tools on the CFIA’s website were very useful in understanding the SFCR. These stakeholders requested that the CFIA continue offering this type of support during SFCR implementation.
CFIA Response:
The CFIA will expand its repository of guidance materials and will make them available for industry to review prior to the publication in the Canada Gazette, Part II.
Regulatory cooperation
The Regulations will allow Canada to keep pace with food regulatory modernization initiatives being pursued by its trading partners, in particular with the United States, which is Canada’s largest export market for food. There is good alignment between the approaches in the Regulations and in the FSMA rules. For example, both regulatory frameworks note the importance of broadly applied preventive approaches including the requirement for preventive controls and written preventive control plans, and both frameworks recognize the primary role that industry plays in the preparation and import of safe food. Also, food businesses in both countries will be required to have a licence or registration, have good manufacturing practices, have traceability requirements (which are aligned with Codex), perform hazard analysis, establish preventive controls, and conduct monitoring. Finally, both the United States and Canada will help small businesses in meeting the new food safety requirements of new regulations by providing assistance through plain language guidance documents, interactive decision tools, and phased-in application dates. The concurrent nature of work on FSMA and work on the Regulations has allowed the CFIA to use this as an opportunity to align our approaches with those of the United States or to minimize differences where it was possible and appropriate to do so.
Some differences exist, namely in the scope of the application of the rules. The U.S. rules apply to all food producers — including those whose product remains in the same state or is sold locally. The Regulations will generally only apply to businesses that import food or prepare food for export or interprovincial trade, and will not apply to those food producers that trade solely within a province (with the exception of some provisions) as they are governed by provincial/territorial authorities. The FSMA also does not apply to those products that are regulated by the U.S. Food Safety Inspection Service (FSIS) [FSIS has a mandate only for meat, poultry, and certain egg products], whereas the SFCR will apply to these products that fall under the CFIA’s mandate.
In general, the outcomes of the SFCR and FSIS regulatory frameworks for meat and processed eggs are aligned. Although the U.S. regulations are more prescriptive in how these outcomes must be met, both countries require HACCP based systems for meat. Proposed amendments to the U.S. regulations for processed eggs also include HACCP implementation. Canadian exporters will still have access to the U.S. market as the SFCR provides the ability to meet both domestic and the U.S. requirements but will also provide the flexibility to continuing meeting the U.S. requirements as they modernize their inspection systems as well as meeting the requirements of other major trading partners.
The SFCR and the FSMA’s requirements provide generally similar exemptions. The FSMA provides exemptions for specific business types (e.g. restaurants, food retail establishments, certain farms) and exempts certain products (e.g. alcoholic beverages, certain fresh fruits and vegetables that are rarely consumed raw, and raw agricultural products). These exemptions are generally consistent with those in the SFCR (e.g. alcoholic beverages, food additives, grains, oilseeds).
The FSMA rules offer “modified requirements” to “very small businesses” or facilities that average less than US$500,000 in annual sales and that sell over half of their production to “qualified end-users” (i.e. direct to consumers, restaurants, retail establishments) not more than 275 miles away. In place of a documented PCP, these facilities must attest to the FDA that they have identified hazards and implemented preventive controls, and continue to monitor them and retain appropriate documents.
In defining “very small businesses” the Food and Drug Administration (FDA) noted that the results of its study of the U.S. food processing sector revealed that even in the smallest category of businesses in the U.S. processed food sector (i.e. those with fewer than 20 employees), nearly all had substantial annual sales that exceed $1 million.footnote 31 The FDA noted that its goal in establishing this definition was to exempt only a small percentage of U.S. food from coverage of this rule in order to minimize the risk of food-borne illness. In light of this, the FDA concluded that a “very small business” definition of business with sales under $1 million was appropriate because it exempts less than 1% of the dollar value of food produced in the United States.footnote 32 Thus, the modified requirements are only available to the very smallest businesses and account for 0.6% of annual U.S. food sales.footnote 33
The SFCR is well aligned with this goal of offering the exception to less than 1% of the dollar value of food produced and imported in Canada in order to offer Canadians the same level of food safety protection. In offering an exception from a written PCP to those businesses with sales less than $100,000, the CFIA will be excepting less than 1% of the dollar value of food produced and imported in Canada from the SFCR’s full suite of food safety requirements. If the CFIA were to adopt the U.S. threshold (i.e. $1 million in annual sales), it would exempt more than 1% of the dollar value of food produced and imported in Canada from the full application of the SFCR. This would exceed both the Canadian and U.S. food safety targets and create a misalignment in food safety outcomes between the SFCR and FSMA.
The requirements described in the Regulations will also help to sustain a major achievement under the RCC, the Food Safety Systems Recognition Arrangement. This arrangement between the U.S. FDA, Health Canada, and the CFIA was signed in April 2016 and it recognizes that the U.S. and Canadian food safety control systems provide a similar level of public health protection. By recognizing each other’s systems, the U.S. FDA and Canada are expressing that they have confidence that they can leverage each other’s science-based regulatory systems. For example, the agreement allows the importing country to consider the exporting country’s comparable level of oversight.
The Regulations will also better align Canada with international approaches by increasing the consistency of the application of preventive approaches through preventive controls, preventive control plans and traceability requirements in a way that focuses on preventing food safety incidents and recognizes the primary role that industry plays in the preparation and import of safe food. The more outcome-based nature of the Regulations will set the stage for a more fundamental discussion with the provinces and territories to achieve domestic equivalence.
The changes introduced in the Regulations are important for maintaining this arrangement as they will keep the Canadian and U.S. approaches comparable.
Rationale
Canada has one of the best food safety systems in the world, but this system must continue to adapt and improve as the food safety environment evolves. Approximately 82 000 businesses of all sizes are deeply integrated into supply chains that prepare and import Canada’s food. In these integrated chains, smaller businesses often supply foods used as ingredients by larger businesses and problems can occur at any stage of preparation (e.g. prior to import, during preparation, during distribution). In such a system, when problems do occur they can quickly become widespread geographically and affect multiple sectors. At the same time, consumers are demanding more information in order to make informed decisions about the foods they purchase.
Industry integration is observed in many countries and foreign regulators and international standard-setting bodies (i.e. Codex) are increasingly advocating the use of systems-based, preventive approaches that identify potential hazards to foods and appropriate controls in order to prevent food safety problems before they occur. They are also advocating the adoption of other practices, such as record keeping, to help recall food products from the market quickly in the event of a food safety incident.
The Regulations will address changes to risks and changes in business practices by establishing a modern and robust legislative framework for food that is prepared in Canada or imported into Canada. The Regulations will also provide new authorities to prevent food safety incidents, respond quickly when incidents occur, and maintain market access.
The Regulations will also better align Canada with international approaches by increasing the consistency of the application of these principles and approaches in Canada in a way that focuses on preventing food safety incidents and recognizes the primary role that industry plays in the preparation and import of safe food. For example, Codex-based guidance on traceability will be applied to a broader range of food businesses. This will address the risk from situations where recall is hindered because a business is unable to provide information regarding where that food originated and where it was sent.
Codex guidance suggesting the broad application of preventive approaches is reflected in the requirement for preventive controls and PCPs (subject to certain exceptions) in the Regulations. These requirements will apply to certain food businesses importing food, or preparing food for export or for interprovincial trade. The choice to broadly apply these approaches also recognizes the integrated nature of Canadian food supply chains that integrate businesses conducting activities representing different levels of risk. The requirements in the Regulations also reflect the lessons learned from previous food safety incidents. Other means of carrying out compliance verification and enforcement such as increased sampling and testing, are more intrusive and costly and will reduce industry accountability.
While the non-federally registered food sector and the fresh fruit and vegetable sector have not been subject to preventive control requirements in the past, many within these sectors have already adopted preventive controls and traceability measures through voluntary programs. That said, there is still a significant number that have not and the Regulations will have the greatest effect on these businesses — which are often small.
Small business activities are often simple and have few controls to implement. In these cases it is relatively simple for an inspector to verify that the business poses a lower food safety risk and is meeting its preventive control requirements. As a business increases in size (i.e. as revenues increase), its activities often become more complex (i.e. they conduct more sophisticated operations, increase their volume of production, increase their number of employees). This increased complexity makes it difficult to perform effective inspections without written documentation, such as a PCP. Overall, this community of small businesses has indicated in the CFIA’s 2017 public opinion research that it has high confidence in its ability to implement the requirements of the SFCR (this was expressed by 83% of respondents).
This difference in complexity and its associated risks have led the CFIA to provide an exception to the PCP requirements for certain very small and micro-businesses with gross annual sales of $100,000 or less that sell inter-provincially, import or export. This provides an exception for a number of businesses (i.e. around 11 240 out of approximately 82 000 total food businesses) that does not substantially weaken the effectiveness of the PCP requirement (i.e. increase food safety risks).
In addition, to help mitigate the costs of new requirements and to promote the uptake of industry best practices among small businesses, the CFIA will provide guidance materials to enhance compliance including “model systems,” plain language guidance documents, PCP templates, and staggered coming-into-force dates for certain requirements in certain sectors that will be included in the Regulations.
The Regulations will also streamline existing requirements to reduce the potential for inadvertent differences and duplications. For example, modification of commodity-specific requirements for meat products that are a mix of ready-to-eat meat products and other non-meat ingredients (e.g. frozen pepperoni pizza) will reduce duplicate requirements. In this situation the ready-to-eat meat in the mixture will already have been subject to food safety requirements earlier in the chain of preparation and the mixture will not be subject again to requirements for the ready-to-eat meat product when it is incorporated into the final food. Streamlining will also address non-food safety requirements (e.g. standards of identity) in a more consistent manner that is flexible enough to accommodate new industry practices.
As other countries modernize their food safety requirements, Canada will need to demonstrate that comparable domestic requirements are in place to maintain market access. This is important given that Canada exported approximately $25.4 billion of food in 2013, a 31% increase from 2009, and exports are significant contributors to the Canadian economy and the Canadian food industry, which is valued at approximately $87.9 billion.
Overall, the Regulations will have several benefits. For consumers, a broader range of foods sold in Canada will be subject to requirements that focus on preventing food safety risks and enable a faster response in the event of a food safety emergency. Beyond a more effective and efficient inspection system, the Regulations are expected to yield cost savings to governments and Canadians through a reduction in food-borne illnesses and costs to the health care system. In addition, implementing the Regulations will be the most cost-effective approach for Government as inspectors will be designated under one act, rather than four (i.e. the CAPA, FIA, MIA and CPLA) and will be trained to a consistent inspection approach enabling deployment to the sectors of highest risk.
Finally, industry will benefit from increased consumer confidence in their products, enhanced market access opportunities through regulatory alignment with major trading partners and less-costly or fewer investigations and recalls. Overall, it is estimated that industry will derive a net benefit from the new streamlined licensing system and more targeted efficient recalls.
Implementation, enforcement and service standards
After the approximately six months delayed coming into force, the CFIA will follow a phased-in implementation approach of the Regulations that reflects the different levels of industry readiness and the concerns of small businesses. Table 1 provides an overview of the phased implementation.
Table 1. Overview of phased implementation timelines
| Meat, Fish, Eggs, Processed Egg, Dairy, Processed Fruit or Vegetable Products, Honey, Maple products |
Fresh Fruits or Vegetables |
All Other Foods footnote 34 |
|||
|---|---|---|---|---|---|
| >$100K and ≥5 Employees |
>$100K and <5 Employees |
≤$100K |
|||
Licence footnote 36 |
Immediately |
+ 1.5 years |
+ 1.5 years |
+ 1.5 years |
|
Traceability |
Immediately (+1 year for growers and harvesters of fresh fruits or vegetables) |
+ 1.5 years |
+ 1.5 years |
+ 1.5 years |
|
Preventive controls footnote 36 |
Immediately |
+ 1 year |
+ 1.5 years |
+ 2.5 years |
+ 2.5 years |
Written PCP footnote 37 |
Immediately |
+ 1 year |
+ 1.5 years |
+ 2.5 years |
Not required footnote 35 |
The CFIA will maintain open and transparent communication with stakeholders to facilitate the transition and implementation period for the Regulations through the CFIA website, and through Ask CFIA (a new service offering a single point of entry for stakeholders to ask questions regarding regulatory requirements).
When the SFCA comes fully into force, it will repeal the CAPA, the FIA, the MIA and amend the CPLA to no longer apply to food. Two federal legislative regimes within the CFIA mandate will apply to food in Canada — the FDA and the SFCA. Food prepared for sale only within provinces will continue to be subject to the requirements of the FDA that generally apply to all food in Canada and to some requirements of the SFCA.
Implementation of the Regulations will be supported by the following:
- new plain language guidance documents and compliance promotion tools that will facilitate the understanding and meeting the regulatory requirements;
- the outcomes of a review (including consultations) on the CFIA’s service standards and service fees;
- continued proactive, multi-channelled communication and engagement with stakeholders to raise awareness, understanding and to prepare them for implementation;
- a new Learning and Training Architecture for food inspectors;
- a new science-based approach to risk rating of foods;
- a modernized and integrated approach to inspection;
- modern IM/IT systems and tools;
- a new performance measurement framework that considers a range of systemic indicators and accountabilities; and
- increased emphasis on a government-industry partnership on food safety.
Compliance and enforcement
The CFIA uses a range of tools to verify compliance, including inspections, surveillance, sampling, and testing. When non-compliance is determined, the CFIA may take enforcement action commensurate with the seriousness of the non-compliance. Under the Regulations, the Minister may suspend or cancel a licence. For example, a licence maybe suspended immediately, upon notice, where there is a risk of injury to human health. This enforcement tool may be taken in addition to other compliance and enforcement tools and measures that are available including food product seizure and detention, an order to remove an imported product from Canada, a recall order, and/or penalties such as the issuance of an administrative monetary penalty under the Agriculture and Agri-Food Administrative Monetary Penalties Act.
Performance measurement and evaluation
It is expected that the Regulations will improve the ability of the CFIA and regulated parties to prevent and manage food safety risks, better protect consumers, and maintain and expand market access for Canada. The CFIA is developing a single food program approach to measure how well its activities, processes, and services contribute to these outcomes.
The CFIA is developing performance indicators to measure the performance of the Regulations, once they come into force. These indicators will allow the CFIA to monitor and assess whether the Regulations are achieving the goal of increasing food safety in Canada. To date, the following indicators have been proposed for this purpose:
- Increase in the number of CFIA-licensed food manufacturers that have a system in place to promote food safety (target: to be determined) [source: CFIA internal data]; and
- Increase in the number of CFIA-licensed food importers that have a system in place to promote food safety (target: to be determined) [source: CFIA internal data].
Contact
Lyzette Lamondin
Executive Director
Food Safety and Consumer Protection Directorate
Canadian Food Inspection Agency
1400 Merivale Road, Tower 1
Ottawa, Ontario
K1A 0Y9
Telephone: 613-773-6189
Email: CFIA-Modernisation-ACIA@inspection.gc.ca
Small Business Lens Checklist
1. Name of the sponsoring regulatory organization:
2. Title of the regulatory proposal:
3. Is the checklist submitted with a RIAS for the Canada Gazette, Part I or Part II?
☐ Canada Gazette, Part I ☑ Canada Gazette, Part II
A. Small business regulatory design
I |
Communication and transparency |
Yes |
No |
N/A |
|---|---|---|---|---|
1. |
Are the proposed Regulations or requirements easily understandable in everyday language? |
☐ |
☑ |
☐ |
The majority of the provisions of the Regulations are easily understandable in everyday language. Some sections however, are technical in nature. Plain language tools will be developed in everyday, non-technical language and will be targeted to different affected stakeholder groups to explain the regulatory requirements. For example, importers will have guidance tailored to their specific needs, as will businesses who prepare food for interprovincial trade. |
||||
2. |
Is there a clear connection between the requirements and the purpose (or intent) of the proposed Regulations? |
☑ |
☐ |
☐ |
3. |
Will there be an implementation plan that includes communications and compliance promotion activities, that informs small business of a regulatory change and guides them on how to comply with it (e.g. information sessions, sample assessments, toolkits, websites)? |
☑ |
☐ |
☐ |
4. |
If new forms, reports or processes are introduced, are they consistent in appearance and format with other relevant government forms, reports or processes? |
☑ |
☐ |
☐ |
II |
Simplification and streamlining |
Yes |
No |
N/A |
1. |
Will streamlined processes be put in place (e.g. through BizPaL, Canada Border Services Agency single window) to collect information from small businesses where possible? |
☑ |
☐ |
☐ |
2. |
Have opportunities to align with other obligations imposed on business by federal, provincial, municipal or international or multinational regulatory bodies been assessed? |
☑ |
☐ |
☐ |
3. |
Has the impact of the proposed Regulations on international or interprovincial trade been assessed? |
☑ |
☐ |
☐ |
4. |
If the data or information, other than personal information, required to comply with the proposed Regulations is already collected by another department or jurisdiction, will this information be obtained from that department or jurisdiction instead of requesting the same information from small businesses or other stakeholders? (The collection, retention, use, disclosure and disposal of personal information are all subject to the requirements of the Privacy Act. Any questions with respect to compliance with the Privacy Act should be referred to the department’s or agency’s ATIP office or legal services unit.) |
☐ |
☐ |
☑ |
The data or information, other than personal information, required to comply with the proposed Regulations is not already collected by another department or jurisdiction. |
||||
5. |
Will forms be pre-populated with information or data already available to the department to reduce the time and cost necessary to complete them? (Example: When a business completes an online application for a licence, upon entering an identifier or a name, the system pre-populates the application with the applicant’s personal particulars such as contact information, date, etc. when that information is already available to the department.) |
☐ |
☑ |
☐ |
The type of information currently collected by the CFIA varies by food program and differs from the information that will be required under the regulatory proposal. However, the information will be pre-populated for renewal or in the case of amendment or application for other licences or permissions granted by the CFIA. |
||||
6. |
Will electronic reporting and data collection be used, including electronic validation and confirmation of receipt of reports where appropriate? |
☑ |
☐ |
☐ |
7. |
Will reporting, if required by the proposed Regulations, be aligned with generally used business processes or international standards if possible? |
☑ |
☐ |
☐ |
8. |
If additional forms are required, can they be streamlined with existing forms that must be completed for other government information requirements? |
☐ |
☐ |
☑ |
The forms required under the proposed Regulations cannot be streamlined with existing forms required for other government information requirements, as the information required is not currently being collected. |
||||
III |
Implementation, compliance and service standards |
Yes |
No |
N/A |
1. |
Has consideration been given to small businesses in remote areas, with special consideration to those that do not have access to high-speed (broadband) Internet? |
☑ |
☐ |
☐ |
2. |
If regulatory authorizations (e.g. licences, permits or certifications) are introduced, will service standards addressing timeliness of decision making be developed that are inclusive of complaints about poor service? |
☑ |
☐ |
☐ |
3. |
Is there a clearly identified contact point or help desk for small businesses and other stakeholders? |
☑ |
☐ |
☐ |
B. Regulatory flexibility analysis and reverse onus
IV |
Regulatory flexibility analysis |
Yes |
No |
N/A |
|---|---|---|---|---|
1. |
Does the RIAS identify at least one flexible option that has lower compliance or administrative costs for small businesses in the small business lens section? Examples of flexible options to minimize costs are as follows:
|
☑ |
☐ |
☐ |
2. |
Does the RIAS include, as part of the Regulatory Flexibility Analysis Statement, quantified and monetized compliance and administrative costs for small businesses associated with the initial option assessed, as well as the flexible, lower-cost option? |
☑ |
☐ |
☐ |
3. |
Does the RIAS include, as part of the Regulatory Flexibility Analysis Statement, a consideration of the risks associated with the flexible option? (Minimizing administrative or compliance costs for small business cannot be at the expense of greater health, security or safety or create environmental risks for Canadians.) |
☑ |
☐ |
☐ |
4. |
Does the RIAS include a summary of feedback provided by small business during consultations? |
☑ |
☐ |
☐ |
V |
Reverse onus |
Yes |
No |
N/A |
1. |
If the recommended option is not the lower-cost option for small business in terms of administrative or compliance costs, is a reasonable justification provided in the RIAS? |
☐ |
☐ |
☑ |
The recommended option is the lower-cost option. |
||||