Canada Gazette, Part I, Volume 148, Number 32: Hazardous Products Regulations
August 9, 2014
Statutory authority
Hazardous Products Act
Sponsoring department
Department of Health
REGULATORY IMPACT ANALYSIS STATEMENT
(This statement is not part of the regulations.)
Executive summary (see footnote 1)
Issues: The Government of Canada is proposing to revise the classification and hazard communication requirements related to workplace hazardous chemicals (see footnote 2) in order to align the current system with that of the United States and other key trade partners. This is expected to reduce costs for industry while simultaneously enhancing the health and safety of Canadian workers. Despite the substantial integration of the Canadian and U.S. markets, and generally similar risk tolerances in areas related to workplace health and safety in both countries, regulatory differences continue to hinder two-way trade in areas such as workplace hazardous chemicals. In addition, expanding global trade in this area makes it increasingly complex to maintain clear, consistent, and easily accessible information for workers. The United States, along with many of Canada's other trading partners, are now in the process of implementing the new global standard for the classification and labelling of workplace hazardous chemicals known as the Globally Harmonized System for the Classification and Labelling of Chemicals (GHS). The results of analysis and consultations suggest that not moving to the international standard in this area would result in increased costs for industry; growing difficulty in ensuring consistent and coherent hazard information is provided to employers and workers; and negative trade consequences for Canadian companies operating in this sector.
Description: This regulatory proposal includes three sets of changes. Firstly, it would implement the initiative announced as part of the Canada–United States Regulatory Cooperation Council (RCC) Action Plan in December 2011 to implement the new GHS for Canada's workplace hazardous chemicals sector, in alignment with the United States. Fulfilling this RCC commitment requires the repeal of the Controlled Products Regulations (CPR) and their replacement with new the Hazardous Products Regulations (HPR) so as to implement the GHS classification and hazard communication standard. The proposed regulations would substantially harmonize Canadian classification and hazard communication requirements for workplace hazardous chemicals with those of the United States and other jurisdictions that have implemented the GHS. Secondly, these changes would necessitate consequential amendments to the following regulations: Food and Drug Regulations; Hazardous Materials Information Review Regulations, Hazardous Materials Information Review Act Appeal Board Procedures Regulations, Consumer Chemicals and Containers Regulations, 2001, and Safety of Human Cells, Tissues and Organs for Transplantation Regulations. There would also be changes in the following two regulations made under the Canadian Environmental Protection Act, 1999: (i) New Substances Notification Regulations (Chemicals and Polymers), and (ii) Export of Substances on the Export Control List Regulations. Thirdly, the proposal would amend the Hazardous Materials Information Review Regulations and the Hazardous Materials Information Review Act Appeal Board Procedures Regulations to reflect changes to the Hazardous Materials Information Review Act that came into force on April 1, 2013, as a result of the Jobs and Growth Act, 2012.
Cost-benefit statement: The adoption of this regulatory package is expected to result in health and safety benefits for Canadian workers, including fewer personal injuries, fewer acute and chronic illnesses, and fewer fatalities. While there would be costs associated with adapting to the new system in the first few years of implementation, including the costs for reclassification and training, it is estimated that there would be net benefits for industry in the medium and long terms. Over a 20-year period, costs to industry are estimated at $285.5 million (present value), and benefits are estimated at $687.5 million (present value). This will yield estimated benefits of $391.6 million (net present value). In addition, although they have not been fully quantified, it is also assumed that there would be substantial benefits resulting from decreased barriers to trade.
“One-for-One” Rule and small business lens: The “One-for-One Rule” does not apply to this regulatory proposal. The proposal to implement the GHS does not include requirements for industry to demonstrate compliance with the proposed regulations, such as collecting, processing, reporting and retaining information or completing of forms. As a result, the proposed regulations do not place an administrative burden on industry, and, therefore, the “One-for-One Rule” does not apply. Similarly, the two sets of consequential amendments described above are related only to updating definitions and terminology; there is no change, for example, to the processes associated with claims to protect confidential business information in the Hazardous Materials Information Review Regulations and the Hazardous Materials Information Review Act Appeal Board Procedures Regulations. These changes do not have an effect on administrative burden.
Given that the proposed regulations are expected to result in an overall reduction in costs borne by small businesses, the small business lens does not apply.
Domestic and international coordination and cooperation: This initiative would implement the new global standard for the classification and labelling of workplace chemicals in alignment with the approach being implemented in the United States. It is also designed to maintain a collaborative approach with provincial and territorial partners as well as industry and worker representatives.
Background
Canada's workplace chemicals hazard communication system — the Workplace Hazardous Materials Information System (WHMIS) — has been in place since 1988. WHMIS is a comprehensive system for providing health and safety information to promote the safe use of hazardous chemicals used in Canadian workplaces.
WHMIS is implemented through interlocking federal, provincial and territorial legislation. At the federal level, the Hazardous Products Act (HPA) and the Controlled Products Regulations (CPR) require the suppliers of hazardous chemicals intended for workplace use to classify these products and provide related hazard information through labels and material safety data sheets (MSDSs). In the workplace, federal (through the Canada Labour Code), provincial and territorial occupational safety and health (OSH) legislation and regulations set out requirements for employers to inform and train employees with regard to the safe handling, storage and use of hazardous chemicals in the workplace. In addition, under the federal Hazardous Materials Information Review Act, suppliers regulated under the HPA as well as employers regulated under federal, provincial or territorial OSH legislation can make claims for exemption from disclosure of confidential business information. While this work used to be done by the Hazardous Materials Information Review Commission, the Jobs and Growth Act 2012 transferred the Commission's responsibilities and functions to Health Canada.
Tens of thousands of new or reformulated workplace hazardous chemicals enter the Canadian market every year. This is in addition to the hundreds of thousands of workplace hazardous chemicals already offered for sale to, and in use in, Canadian workplaces. While the exact number of products regulated under WHMIS is not fully known, there is evidence to suggest that there are over 300 000 products covered by WHMIS. (see footnote 3) According to Industry Canada, the Canadian chemical manufacturing sector imported more than $28 billion worth of chemicals from the United States in 2013 and exported more than $24 billion to the United States. (see footnote 4) These trade volumes represent only a small fraction of the total value of substances used in Canada to which the WHMIS program applies.
In response to the challenges faced by suppliers, employers and workers due to the lack of international alignment in the classification, labelling and provision of safety information for workplace hazardous chemicals, Canada, the United States and other countries worked together under the auspices of the United Nations over the past two decades to develop the Globally Harmonized System of Classification and Labelling of Chemicals (GHS). The GHS was endorsed as a global standard by the United Nations General Assembly in 2003 for four hazard communication systems: the transportation of dangerous goods, (see footnote 5) consumer products, pest control products and workplace chemicals. The United States, the European Union (EU), Australia, New Zealand, China, Japan, South Korea and others have either applied, or are in the process of applying, the GHS to their workplace hazardous chemicals hazard communication systems.
The Canada–United States Regulatory Cooperation Council (RCC) was announced in February 2011 by the Prime Minister of Canada and the President of the United States. One of its key objectives is the facilitation of trade between the two countries. In December 2011, one of 29 initiatives announced as part of the RCC Joint Action Plan was the coordinated implementation of the GHS for workplace hazardous chemicals. Specifically, Canada and the United States agreed to “align and synchronize implementation of common classification and labelling requirements for workplace hazardous chemicals within the mandate of the U.S. Occupational Safety and Health Administration (OSHA) and Health Canada (HC).”
The United States are now in the process of applying the GHS to that country's hazard communication system for workplace hazardous chemicals. As a result, through the RCC initiative, Canada has a unique opportunity to substantially align its workplace hazardous chemicals regulatory regime with that of its major trading partner, as well as achieve significant alignment with other trading partners (e.g. the European Union, Australia, China, Japan, South Korea) that are also in the process of implementing the GHS. This initiative would enable the modernization of a complex regulatory regime that has remained largely unchanged since it was put in place in Canada in 1988. It would also reduce unnecessary administrative costs for both the suppliers of workplace hazardous chemicals and the employers that use them.
Issues
Currently, the rules and regulations for classification and labelling of workplace hazardous chemicals differ from one country to another. For example, despite the substantial integration of Canadian and U.S. markets and generally similar risk tolerances in areas related to workplace health and safety in both countries, regulatory differences continue to hinder two-way trade in workplace hazardous chemicals. Canadian companies in the business of selling workplace hazardous chemicals internationally carry significant costs because they are required to classify and prepare labels and safety data sheets (SDSs) (see footnote 6) according to the regulations of each country in which their products are sold.
For Canadian companies exporting workplace hazardous chemicals to the United States, the lack of common classification and labelling criteria necessitates the reclassification and relabelling of these products. The cost and time associated with reclassification and relabelling hinder the ability of Canadian companies to market their products in the United States. In addition, the differences in classification criteria between the two systems can prevent test data that was used for classification in Canada from being applicable in the United States. This results in the potential need for retesting and reclassification and entails additional costs.
Similarly, U.S. exporters of workplace hazardous chemicals to Canada must also reclassify and relabel their products to comply with Canadian standards, and the associated costs hinder these companies from marketing their products in Canada. The increased burden on U.S. companies results in limited access to these products in Canada or in higher prices paid by the Canadian importer. The additional costs are passed on to Canadian distributors and, eventually, to the employers that purchase these products for use in their workplaces. In cases where the U.S. company has not classified and labelled the product based on Canadian requirements, the Canadian importer bears the cost of reclassifying and relabelling the product.
While it is not possible to quantify or monetize the total costs associated with reclassifying and preparing new labels and SDSs as a result of the different requirements between Canada and the United States, suppliers have indicated that this situation creates significant costs for Canadian businesses. For example, one small Canadian company indicated that it would save over $20,000 a year in relabelling costs alone if Canada's requirements were harmonized with those of the United States. In another example, one industry association indicated that, if hazard communication systems were not harmonized between Canada and the United States, one of its members in the specialty chemical products industry would face costs of $2.5 million in creating labels that comply with both systems.
Maintaining the current classification and information regime for workplace hazardous chemicals in Canada is increasingly placing Canadian companies at a disadvantage given that the vast majority of Canada's trading partners have applied, or are in the process of applying, the GHS to their workplace hazardous chemicals programs. Canadian industry is not currently able to benefit from the reduced costs and substantial future savings that would result from factors such as not having to reclassify and prepare different labels and SDSs for workplace hazardous chemicals being imported from, or exported to, countries that have adopted the GHS approach.
Applying the GHS would also enhance worker health and safety through the provision of more consistent hazard information. In addition, the GHS would cover some types of hazards that are not currently covered in Canada; this would further enhance protections for workers. The number of accidents and injuries is expected to decrease as a result of this proposal. Currently, Canadian employers and workers are not benefitting from the health and safety advantages that employers and workers in the jurisdictions noted above are afforded by a globally recognized standard for classifying workplace hazardous chemicals and communicating how to better ensure their safe use.
The regulatory requirements for classification and labelling under WHMIS have remained largely unchanged since they were established in 1988. While WHMIS provides for an integrated and comprehensive process for identifying and communicating the hazards associated with workplace hazardous chemicals, there are aspects that could be strengthened and refined to improve the clarity and consistency of the hazard information provided to Canadian workers.
The GHS contains many elements that represent improvements on the current WHMIS, for example,
- the GHS hazard classification criteria are more comprehensive and detailed than those currently in WHMIS, which improves the ability to indicate the severity of hazards;
- the GHS identifies and addresses hazards not currently addressed in WHMIS (e.g. specific target organ toxicity — single exposure and aspiration hazard);
- the GHS hazard definitions and classification criteria are consistent with other hazard communication systems already in use in Canada (e.g. the physical hazard criteria with respect to the transportation of dangerous goods are already harmonized with the GHS);
- the GHS provides for specific language to convey hazard information, and, as a result, employers and employees are given the same core information on a chemical regardless of the supplier, and the standardization of the language would improve the comprehensibility of the hazard information;
- some of the GHS pictograms are more easily comprehensible and are anticipated to improve hazard communication, particularly for workers who are not functionally literate, who are not literate in the language used on the label, or who have experience working in other international jurisdictions;
- while the GHS format for SDSs has been allowed for use in Canada through an administrative policy, requiring the standardized GHS format would help to ensure that information is easier for users to find as it would be presented in a consistent manner across all SDSs and the information that employees and emergency responders need most appears in the beginning of the document for easy identification and reference; and
- the standardized GHS SDS information requirements are more comprehensive and therefore provide employers and employees with a broader scope of information related to a workplace hazardous chemical, which improves employers' ability to train and educate workers.
Objectives
The implementation of this regulatory proposal is intended to align and synchronize Canada's application of the GHS for its workplace hazardous chemicals system with the approach taken in the United States in order to achieve the following objectives:
- facilitate trade through common labelling and other hazard communication requirements;
- lower costs for businesses and consumers by reducing the need for retesting and reclassifying workplace hazardous chemicals from, or for, different markets; and
- increase worker protections through the adoption of an improved, globally recognized standard for communicating the hazards associated with workplace hazardous chemicals.
Description
These regulatory changes would affect the industries that sell and import workplace hazardous chemicals, the employers and workers who work with workplace hazardous chemicals, and the government bodies that regulate workplace hazardous chemicals (Health Canada, as well as federal, provincial and territorial occupational health and safety agencies).
Under the authority of an amended Hazardous Products Act, it is proposed that the Controlled Products Regulations (CPR) be repealed and replaced with new regulations to be titled the Hazardous Products Regulations (HPR). These new regulations would implement the Globally Harmonized System for the Classification and Labelling of Chemicals (GHS) hazard classification criteria and hazard communication elements — labels and safety data sheets (SDSs) — as per the fifth revision of the GHS published by the United Nations in 2013. In addition, the proposed regulations are, to the maximum extent possible, in alignment with the U.S. Occupational Safety and Health Administration (OSHA) Hazard Communication Standard (HCS) as amended on March 26, 2012 (HCS 2012).
The GHS elements are proposed for adoption in a manner that is consistent with the GHS “building block” approach. The GHS may be considered as a collection of “building blocks,” such as hazard classes or divisions within a hazard class, that are available for adoption by a jurisdiction. Additionally, the proposed regulations stipulate that workers would continue to be provided with health and safety information in respect of workplace hazardous chemicals to the same or to a greater extent than is currently the case under the CPR. Lastly, the proposal maximizes alignment between the proposed HPR and the OSHA HCS 2012 with the exception of areas where a variance is necessary to maintain the current level of protection afforded to Canadian workers or to respect the framework of the Canadian legislation and regulations.
The proposed HPR would repeal and replace the CPR and would repeal the Ingredient Disclosure List. The proposed regulations would differ from the CPR in five broad areas: (1) the manner of establishing the classification of workplace hazardous chemicals; (2) classification of physical hazards; (3) classification of health hazards; (4) hazard communication and other requirements; and (5) exemptions. These changes would also necessitate consequential amendments to the following regulations: Food and Drug Regulations; Hazardous Materials Information Review Regulations; Hazardous Materials Information Review Act Appeal Board Procedures Regulations; Consumer Chemicals and Containers Regulations, 2001; New Substances Notification Regulations (Chemicals and Polymers); Safety of Human Cells, Tissues and Organs for Transplantation Regulations; Export of Substances on the Export Control List Regulations.
In addition, consequential amendments to the Hazardous Materials Information Review Regulations and Hazardous Materials Information Review Act Appeal Board Procedures Regulations would be required in relation to amendments that were made to the Hazardous Materials Information Review Act, through the Jobs and Growth Act, 2012, that came into force on April 1, 2013.
The following provides a brief description of the key regulatory changes proposed in each of these areas, as well as a summary of the rationale supporting the proposals.
(1) Manner of establishing the classification of workplace hazardous chemicals
A new approach to establishing the classification of workplace hazardous chemicals would be set out in the HPR by integrating the relevant provisions of the GHS, as adopted by OSHA, with the current manner of establishing classification under the CPR. In general, the new manner of establishing classification would be similar to the existing manner in that all relevant available data would have to be considered. The CPR principle that classification should be based on available data and no testing should have to be undertaken for the purposes of classification would be retained and is harmonized with the GHS and HCS 2012. However, the proposed GHS approach to the classification of mixtures is more structured than that in the current CPR as it provides a stepwise approach to the consideration of different types of data available for a mixture or its ingredients. The classification of substances would be based on the evaluation of the substance, using all available data, against the criteria for each hazard class.
In addition, a new provision in the proposed regulations would allow the classification of substances to be prescribed in regulation. This is a means of ensuring that substances currently classified under the CPR remain classified under the HPR, despite small differences in the classification criteria between the CPR and the HPR. Where a classification is prescribed for a substance, the substance would nonetheless need to be evaluated against the classification criteria of all other hazard classes.
Two types of GHS hazard classes are proposed for adoption in alignment with HCS 2012: physical hazard classes, which represent hazards relating to physical and chemical properties, such as flammability or compressed gases, and health hazard classes, which represent hazards to health arising from exposure to a substance or mixture, such as acute toxicity or skin sensitization. With respect to the physical hazard classes, the same manner of establishing classification would be used for both substances and mixtures. With respect to the health hazard classes, the manner of establishing classification of mixtures would follow the GHS procedures for each hazard class. These include the application of bridging principles that allow, amongst other things, a classification to be determined for an untested mixture on the basis of a similar tested mixture, as well as the classification of the mixture on the basis of its ingredients subject to consideration of the concentrations of the ingredients and their interactions in the mixture. Classification within these two types of hazard classes is described below.
The proposed regulations would specify that a workplace hazardous chemical would need to be classified in the division of the hazard class that represents the greatest hazard for which it meets the classification criteria. However, the Acute Toxicity, Respiratory or Skin Sensitization, Reproductive Toxicity, and Specific Target Organ Toxicity — Single Exposure hazard classes would permit classification in multiple divisions within these hazard classes, where appropriate, in accordance with the GHS building blocks and in alignment with the HCS 2012. This approach would encourage the identification of all relevant hazards.
Different products sold together in one outer container but packaged individually would each be treated as an individual product for the purposes of classification.
The proposed HPR is aligned with the HCS 2012 in respect of all requirements relating to the manner of establishing the classification of a product.
(2) Classification — Physical hazards
The GHS physical hazard classes and their classification criteria are proposed to be adopted in the HPR in alignment with the HCS 2012. While the GHS hazard classes subdivide physical hazards in a manner that differs from the current CPR, these classes generally address all of the physical hazards that are currently covered in the CPR.
The GHS physical hazard classes proposed for adoption in the HPR that are currently covered in the CPR are the following: Flammable Gases; Flammable Aerosols; Oxidizing Gases; Gases under Pressure; Flammable Liquids; Flammable Solids; Self-Reactive Substances and Mixtures; Pyrophoric (see footnote 7) Liquids; Pyrophoric Solids; Self-Heating Substances and Mixtures; Substances and Mixtures that, in Contact with Water, Emit Flammable Gases; Oxidizing Liquids; Oxidizing Solids; Organic Peroxides; and Corrosive to Metals.
In addition, a Physical Hazards Not Otherwise Classified (PHNOC) hazard class is proposed in order to capture some products that are currently covered under the CPR but that are not addressed by the GHS, such as products that undergo vigorous polymerization, (see footnote 8) and to cover any unforeseen hazards.
The proposed HPR also introduces the following new hazard classes that are not addressed in the CPR: Pyrophoric Gases; Simple Asphyxiants; and Combustible Dusts. These hazard classes are not addressed by the GHS but are proposed for adoption in the HPR in alignment with the HCS 2012 so that they will be communicated to workers. Notably, the proposed HPR would not regulate products that are shipped in a non-dust form but which, when processed, would present the hazard of combustible dust. However, the HCS 2012 requires such products to be accompanied by a label and SDS. This difference is not an impediment to harmonization because the voluntary provision of a label or SDS for such products in Canada would not be viewed as non-compliant with the requirements.
The proposed HPR is aligned with the HCS 2012 in respect of all of the physical hazard classes, with the exception of Combustible Dusts and PHNOC. While the manner in which these hazards would be addressed by the HPR and the HCS 2012 would be different, the outcome would be similar. The HCS 2012 neither defines nor provides classification criteria in respect of combustible dusts. It also does not define a hazard class for physical hazards not otherwise classified, but instead, defines the general term “hazards not otherwise classified.” In both cases, the proposed HPR sets out a hazard class that includes a definition and classification criteria; this is required since the criminal law framework of Canadian legislation and regulations for workplace hazardous chemicals does not provide the latitude to require the classification of a product without specifying the criteria by which a supplier must determine whether the product is classified. Health Canada would continue to collaborate with OSHA so that a harmonized definition of combustible dusts would be applied in both jurisdictions.
(3) Classification — Health hazards
The GHS health hazard classes and their classification criteria are proposed to be adopted in the HPR in alignment with the HCS 2012. While the GHS hazard classes subdivide health hazards in a manner that differs from the current CPR, these classes generally address all of the health hazards that are currently covered in the CPR, and introduce some additional types of hazards that are not currently covered but would enhance protections for workers.
The GHS health hazard classes proposed for adoption in the HPR that are currently covered in the CPR are the following: Acute Toxicity (Categories 1 to 4); Skin Corrosion/Irritation (Categories 1A, 1B, 1C and 2); Serious Eye Damage/Eye Irritation (Categories 1, 2A and 2B); Respiratory or Skin Sensitization (Categories 1A and 1B for both respiratory and skin sensitization); Germ Cell Mutagenicity (Categories 1A, 1B and 2); Carcinogenicity (Categories 1A, 1B and 2); Reproductive Toxicity (Categories 1A, 1B, 2 and an additional category for effects on or via lactation); and Specific Target Organ Toxicity — Repeated Exposure (Categories 1 and 2). Notably, substances that react vigorously with water to release a toxic gas (currently classified as a Dangerously Reactive Material under the CPR) would be classified in the Acute Toxicity hazard class of the proposed HPR in alignment with the HCS 2012. The category building blocks proposed for adoption within each hazard class are aligned with the HCS 2012 and provide classification criteria that are as broad as, or broader than, the criteria under the CPR.
The proposed HPR introduces the following GHS hazard classes which are not addressed in the CPR, in alignment with the HCS 2012: Specific Target Organ Toxicity — Single Exposure (Categories 1, 2 and 3); and Aspiration Hazard (Category 1). It also introduces a Health Hazards Not Otherwise Classified (HHNOC) hazard class that is not addressed by the GHS but is proposed for adoption in the HPR in alignment with the HCS 2012. Lastly, the proposed HPR retains a separate hazard class for Biohazardous Infectious Materials in order to maintain the current level of worker protection in Canada. The classification criteria of this class would be amended to align with the definitions of “Risk Group 2,” “Risk Group 3,” and “Risk Group 4” as defined by the Human Pathogens and Toxins Act in order to ensure consistency with that Act.
The proposed HPR is aligned with the HCS 2012 in respect of all of the health hazard classes, with the exception of the Biohazardous Infectious Materials and HHNOC hazard classes. The HCS 2012 does not regulate biohazardous infectious materials; however, the class is proposed to be retained in the HPA to maintain the current level of worker protection in Canada. It is expected that this variance would have a limited impact as biohazardous infectious materials are often specialized products that are distinct from most chemical products, and the market for such products is limited. With respect to the HHNOC hazard class, the HCS 2012 does not define a hazard class, but instead, defines the general term “hazards not otherwise classified” that encompasses health and physical hazards. In the proposed HPR it is divided into two hazard classes — PHNOC and HHNOC — so that there is the appropriate classification of substances or ingredients in a mixture that present a physical or health hazard that is not otherwise addressed by the GHS and is currently covered by the CPR. The proposed HHNOC hazard class in the HPR includes a definition and classification criteria due to the framework of the Canadian legislation and regulations, as described above. It is expected that a very small number of chemicals would be classified in this hazard class, and therefore the different requirements would be expected to impact very few products.
(4) Hazard communication and other requirements
The HPA requires a label and an SDS for each workplace hazardous chemical, i.e. each product that meets classification criteria set out in the HPR. The current requirements for labels and SDSs would be amended to respect the content and format specifications of the GHS, in alignment with the HCS 2012. This includes a proposal that the GHS term “safety data sheet” (SDS) replace the WHMIS term “material safety data sheet” (MSDS). However, the general approach to appropriately communicating the hazards of a product on a label and an SDS through pictograms and statements that convey messages about hazards, precautions and first aid measures would remain the same.
Labelling
Under the HPR, a label is proposed to comprise a product identifier and initial supplier identifier, as well as standardized pictograms, a signal word, hazard statements, precautionary statements and, where applicable, supplemental label elements that are required based on the classification of the workplace hazardous chemical. The GHS pictogram format of a black symbol on a white background with a red frame in the shape of a square set on a point would be adopted for all GHS pictograms. The current CPR symbol for biohazards, in a black circle, would be retained as the biohazard symbol as there is no equivalent symbol under the GHS. For all other hazards, the GHS pictograms would be adopted. The hazard pictogram(s), the signal word and the hazard statement(s) would be required to be grouped together on the label. It would additionally be specified that the label must be durable and legible without the aid of any devices other than corrective lenses. The CPR requires similar label elements (hazard symbols, risk phrases and first aid measures) to convey the hazard to workers. However, the only standardized CPR label element is the hazard symbol, whereas the GHS, HCS 2012 and the proposed HPR also require standardized hazard statements, signal words and precautionary statements.
The initial supplier identifier would be the contact information for the Canadian manufacturer or importer. However, instead of providing the initial supplier identifier, a distributor could provide their contact information if desired and an importer could retain the name of the foreign supplier if the product was imported for the importer's own use. This approach is aligned with the HCS 2012, which requires the disclosure of the identity of a U.S. manufacturer, importer or other responsible person. Each jurisdiction requires the identification of a supplier within its jurisdiction for the purposes of verifying compliance and carrying out enforcement, and thus imported products in both jurisdictions would be required to identify the importer for that jurisdiction.
For each hazard class adopted from the GHS in which the product is classified, the corresponding pictogram, signal word, hazard statement and precautionary statements set out in section 3 of Annex 3 of the GHS would be required to appear on the label. For all other hazard classes in which the product is classified, those elements as set out in the HPR for the hazard class would be required. The GHS supplemental label element warning that a certain percentage of ingredients in a mixture are of unknown acute toxicity would also be adopted and required to appear on the label of such mixtures. Supplemental hazard statements would also be required on labels of products that, upon contact with water, release a gaseous substance that is acutely toxic (i.e. water-activated toxicants). The HPR would contain rules of precedence to prevent the duplication of information on the label. In addition, hazard statements could be combined, as appropriate, as could precautionary statements, and inapplicable precautionary statements could be omitted. Unlike the proposed HPR, the HCS 2012 allows the omission of non-applicable hazard statements. The impact of this variance is, however, expected to be small, because the number of cases in which it would be appropriate to classify a product but not apply the associated hazard statement is expected to be limited.
The requirement from the CPR for a hatched border around the label content is not proposed to be retained, nor is the requirement that the label contain a statement to the effect that a material safety data sheet is available. These requirements are not harmonized with the GHS or the HCS 2012 and their removal is not expected to result in a lowering of the protections available to workers.
In addition to the exceptions noted above, the proposed HPR are not aligned with the HCS 2012 in respect of the following label requirements for the Carcinogenicity, PHNOC, HHNOC and Biohazardous Infectious Materials hazard classes:
- Carcinogenicity — The proposed HPR require, as do the current CPR, a label on all mixtures containing a carcinogenic ingredient at a concentration of 0.1% or more. The HCS 2012 makes a label optional for mixtures containing a Category 2 carcinogen at a concentration between 0.1% and 1%. This option would not maintain the current level of worker protection in Canada and is therefore not proposed to be adopted. It is expected that between 1% and 3% of all labels would be impacted by this variance.
- PHNOC and HHNOC — The proposed HPR require a label on all products classified in these classes whereas the HCS 2012 does not. Because the nature of these hazards, as defined by the proposed HPR, is such that they may cause the death or serious injury of a person in the case of a PHNOC, or death or an adverse effect on a person's health, including an injury, in the case of a HHNOC, label elements were deemed to be necessary. The label elements proposed are a pictogram, a hazard statement and precautionary statements that are appropriate to the hazard, as well as the signal word “Danger.”
- Biohazardous Infectious Materials — Products meeting the criteria in the proposed HPR would be required to be labelled with the biohazard pictogram, the signal word “Danger” and the appropriate hazard statement and precautionary statements. Biohazardous infectious materials are not regulated by the HCS 2012. It is expected that this variance would have a limited impact as biohazardous infectious materials are specialized products that are distinct from most chemical products and the market for such products is limited.
- Substances and mixtures that, upon contact with water, release a gaseous substance that is acutely toxic — The proposed HPR require a supplemental hazard statement on the label of products that meet the criteria for this hazard to maintain the existing standard of protection for workers. The HCS 2012 does not require a supplemental label element. The impact of this variance is expected to be small, because a limited number of products would meet the criteria for this hazard.
Safety Data Sheets (SDSs)
The SDS under the proposed HPR would have a format of 16 standardized GHS headings in alignment with the HCS 2012. Available information with respect to each header/topic would have to appear in the SDS, with the exception that the information in items 12 to 15 would be optional, in alignment with the HCS 2012.
| Existing CPR | Proposed HPR | ||
|---|---|---|---|
| Item | Suggested Heading | Item | Required (GHS) Heading |
| 1 | Hazardous Ingredients | 1 | Identification |
| 2 | Preparation Information | 2 | Hazard identification |
| 3 | Product Information | 3 | Composition/Information on ingredients |
| 4 | Physical Data | 4 | First aid measures |
| 5 | Fire or Explosion Hazard | 5 | Firefighting measures |
| 6 | Reactivity Data | 6 | Accidental release measures |
| 7 | Toxicological Properties | 7 | Handling and storage |
| 8 | Preventive Measures | 8 | Exposure controls/ Personal protection |
| 9 | First Aid Measures | 9 | Physical and chemical properties |
| 10 | Stability and reactivity | ||
| 11 | Toxicological information | ||
| 12 | Ecological information | ||
| 13 | Disposal considerations | ||
| 14 | Transport information | ||
| 15 | Regulatory information | ||
| 16 | Other information | ||
For products classified as Biohazardous Infectious Materials, a new nine-heading appendix to the SDS based on the information sheets made publicly available by the Public Health Agency of Canada is proposed to be required in order to provide additional information that is more specific to the nature of the hazard presented by a biohazardous infectious material. As with the labelling requirements, this requirement would not be harmonized with the United States; biohazardous infectious materials are not regulated by the HCS 2012. However, as described above, this inconsistency should not have a significant impact because biohazardous infectious materials are distinct from most chemical products, and the market for these products is limited.
The SDS would be required to provide, in the case of a material or substance, its chemical name. In the case of a mixture, the chemical name and concentration or concentration range of all ingredients in the mixture that present a health hazard would be required to be disclosed on the SDS. The HPA previously required, in addition to the disclosure of ingredients classified as health hazards, the disclosure of (i) ingredients classified as physical hazards, (ii) ingredients listed in the Ingredient Disclosure List, (iii) ingredients the supplier believed on reasonable grounds may be harmful, and (iv) ingredients for which the toxicological properties were not known to the supplier. However, the requirements listed in (i) through (iv) have not been retained in the HPA as they were not to be harmonized with the GHS or the HCS 2012, and their removal will not result in a lowering of the protections available to workers.
In order to harmonize with the GHS and HCS 2012, the following elements would differ from current CPR requirements. The SDS would be required to provide the classification of the product as well as information about any hazardous reaction product produced as a result of having followed instructions for use provided with the product. The CPR did not previously require such information. In addition, and subject to exceptions in the HPR, the initial supplier identifier and product identifier appearing on the SDS would be required to be the same as those appearing on the label. The CPR previously required this of only the product identifier. These small changes would harmonize with the GHS and HCS 2012 and increase the protections available to workers.
The requirements that the SDS disclose any other hazard information that is available to the supplier with respect to the product or a product that has similar properties would be retained. While this requirement is not explicit in the HCS 2012, its removal from the HPR would constitute a reduction in the level of protections available to workers.
The proposed HPR is aligned with the HCS 2012 in respect of all the SDS requirements, with the exception of the HHNOC and the Biohazardous Infectious Materials hazard classes. The chemical identity of an ingredient classified in the HHNOC hazard class would be required to be disclosed in section 3 of the SDS in order to maintain the current level of protection for workers. This is not required under the HCS 2012. As described above, an appendix to the SDS providing information that is specifically relevant to the biohazard would be required for products classified in the “Biohazardous Infectious Materials” hazard class under the proposed HPR.
Other requirements
Information on the label and SDS would continue to be required to be provided in both English and French in conformity with the requirements of the Official Languages Act, and despite the unilingual HCS 2012 requirements. The information could appear either on a single bilingual SDS or two separate unilingual SDSs. Bilingual labels would continue to be required.
The requirement to provide information to a health professional in the case of an emergency would be retained. However, the requirement that the health professional retain the information that was provided in confidence would be subject to the requirement that the health professional be informed that the information must be kept confidential, except for the purpose for which it was provided.
The requirement to revise the SDS every three years in the absence of new information in respect of the product would no longer be required as it is duplicative of the requirement that an SDS and label be accurate at the time of each sale or importation of the product.
(5) Exemptions
The current regulations include a number of provisions that allow, under specified conditions, (i) an exemption from the requirement of the HPA to provide, obtain or prepare an SDS; (ii) an exemption from the requirement of the HPA to have a product label; or (iii) reduced information on labels and SDSs. In the proposed HPR, some of the current exemptions would be removed, some would be retained without modification (other than amendments required as a consequence of other amendments), some would be retained with modification, and a few new exemptions would be created, as described below.
The legislative and regulatory framework in Canada requires that the HPR specify exemptions that can be applied as a rule, rather than on an individual basis.
The exemption for flavours and fragrances would not be retained in order to harmonize with the HCS 2012. The exemption for a generic SDS would not be retained in the regulations, but such an SDS would not contravene the HPA or HPR as long as the user is not misled by the information it contains. The exemptions for complex mixtures, confidential business information as per the amended Hazardous Materials Information Review Act, and SDSs with the same product identifier would be retained largely as they are set out in the CPR. Each may have small modifications as a consequence of amendments made elsewhere in the regulations.
The CPR bulk shipment exemption would be extended to products sold without packaging of any sort (such as bulk oil) regardless of whether they are shipped. This is harmonized with the HCS 2012. In addition, these products would be exempt from the requirement for a label as all label information would be provided within sections 1 and 2 of the SDS required by the proposed HPR; the purchaser would be able to create a label based on that information.
Products packaged in small volume containers with a capacity of less than 100 mL are proposed to be exempt only from the requirement to bear precautionary or hazard statements on the label. The HCS 2012 does not provide for such an exemption, but OSHA addresses provisions for small package labelling in response to questions from individual suppliers.
Only two of the existing exemptions from the labelling of the outer container of a product would be retained: (1) when the inner container label is visible and legible through the outer container under normal conditions of storage and handling; and (2) when the outer container has a label in accordance with the Transportation of Dangerous Goods Regulations. The HCS 2012 only requires the immediate (innermost) container of a product to be labelled; therefore, it requires no exemption for the labelling of outer containers. However, the requirement to label all containers in which a product is packaged, with the exemptions as proposed, must be retained in order to maintain the current level of worker protection in Canada.
Three of the existing exemptions for radioactive nuclide mixtures are proposed to be retained: (1) non-radioactive carriers present in small quantities (<1 mL or <1 g) and not classified in specified hazard classes need no label or SDS requirements; (2) non-radioactive carriers need no label on the inner container if the outer container bears the required label; and (3) non-radioactive carrier labels do not require the initial supplier identifier and precautionary statements. The exemption for carrier materials such as radioactive drugs or diagnostic devices is not proposed to be retained as these products are excluded from the scope of application of the HPA by section 12. The exclusion for radioactive nuclides in quantities greater than the quantity specified for that nuclide in the Transport Packaging of Radioactive Materials Regulations is not retained as those Regulations no longer exist. The HCS 2012 does not provide exemptions for radioactive nuclide mixtures.
As the HPA now regulates the sale and importation of bailed (see footnote 9) hazardous products, there is an enlargement in the scope of products affected by the exemptions for laboratory samples if not amended. Therefore, it is proposed that a laboratory sample in quantities of less than 10 kg and classified as a Biohazardous Infectious Material would not require an SDS if the sample is imported or sold (whether it is bailed or not), and would not require an SDS or label if the sample is bailed.
Also, it is proposed that a laboratory sample in quantities of less than 10 kg would not require an SDS if the sample is bailed and the chemical name and concentration of the sample or its ingredient are not known, or the sample is a non-commercialized product.
In both previous cases, reduced labelling requirements would also apply. OSHA regulates some laboratory samples under Occupational exposure to hazardous chemicals in laboratories, but provides no exemptions for other laboratory samples.
In addition, the following new exceptions are proposed:
- When bailing a product for the purpose of transportation, the supplier would not need to provide an SDS to the bailee (i.e. the person transporting the product). The provision of hazard information during transportation is covered under the Transportation of Dangerous Goods Regulations.
- Products packaged in a container with a capacity of 3 mL or less where the label interferes with the normal use of the products would be required to have a label that remains durable and legible only while in transport and storage, not during their use. The HCS 2012 does not specifically mention such an exemption, but OSHA addresses provisions for small package labelling when responding to questions from individual suppliers.
- An outer container that contains two or more different products (such as a kit) would be allowed to bear a reduced label. The HCS 2012 does not currently have such an exemption; however, the regulation of kits is still under consideration by OSHA.
- Products that bear a Transportation of Dangerous Goods Regulations pictogram on the label would not require a GHS pictogram for the same hazard. This is aligned with the HCS 2012.
- Chemicals that are not biologically available would not need to be classified in any of the health hazard classes. This is a GHS specification and is aligned with the HCS 2012.
- An SDS and label would be exempt from the requirement to reflect significant new information for a period of 90 and 180 days, respectively, from the date upon which the information became available; this provision is aligned with the HCS 2012. However, the proposed regulations also stipulate that the new information and date upon which it became available must be transmitted by the seller of the product to the person who acquires it, or obtained or prepared by the importer of the product, in written form. This is not required in the United States. The variance between the Canadian and U.S. regulations would have no impact on labels or SDSs. This exemption is proposed as a compromise in order to maintain the current level of protection for workers, respect the nature of the HPA as a criminal statute, and align to the extent possible with the HCS 2012.
(6) Consequential amendments
In relation to the proposed HPR outlined above, consequential amendments are being proposed to the Hazardous Materials Information Review Regulations; the Hazardous Materials Information Review Act Appeal Board Procedures Regulations; the Food and Drug Regulations; the Consumer Chemicals and Containers Regulations, 2001; and the Safety of Cells, Tissues and Organs for Transplantation Regulations. These proposed amendments are consequential in nature and reflect the proposed terminology and definitions of the proposed HPR (e.g. the terminology change from material safety data sheet, or MSDS, to safety data sheet, or SDS). Despite the proposed consequential amendments, the mechanism to protect confidential business information through the Hazardous Materials Information Review Regulations and Hazardous Materials Information Review Act Appeal Board Procedures Regulations would continue to function as it does currently.
Two regulations made under the Canadian Environmental Protection Act, 1999 will also be amended on the recommendation of the Minister of the Environment and the Minister of Health: the Export of Substances on the Export Control List Regulations and the New Substances Notification Regulations (Chemicals and Polymers).
In addition, further consequential amendments to the Hazardous Materials Information Review Regulations and Hazardous Materials Information Review Act Appeal Board Procedures Regulations are being proposed to reflect the amendments to the Hazardous Materials Information Review Act (HMIRA) that came into force on April 1, 2013, as a result of the enactment of the Jobs and Growth Act, 2012. These proposed amendments would reflect the transfer of the powers and functions under the HMIRA from the Hazardous Materials Information Review Commission to Health Canada. The amendments would also align the definitions and terminology of these two regulations with that of the Hazardous Materials Information Review Act; there would be no change to the process for claiming an exemption for confidential business information.
In summary, it is proposed that the current Controlled Products Regulations and the Ingredient Disclosure List be repealed, and new regulations, under the title Hazardous Products Regulations, be made to enable Canada to apply the new globally recognized standard for classifying and communicating hazards to its workplace hazardous chemicals system and to do so in alignment with the approach adopted in the United States in its Hazard Communications Standard as amended on March 26, 2012. It is also proposed that the appropriate consequential amendments be made to other regulations, and that the Hazardous Materials Information Review Regulations and the Hazardous Materials Information Review Act Appeal Board Procedures Regulations be updated to reflect recent changes to the Hazardous Materials Information Review Act as a result of the Jobs and Growth Act, 2012.
Coming into force
In order to align and synchronize the implementation of the GHS for workplace hazardous chemicals with the United States, the proposed regulatory changes would need to come into force no later than June 1, 2015.
Regulatory and non-regulatory options considered
Only a regulatory option would enable Canada to successfully align and synchronize requirements for workplace hazardous chemicals with those of the United States and other major trading partners. As a result, only regulatory options were considered. The following four regulatory options were considered, including the recommended option (i.e. Option 4 below).
Option 1. Status quo — Do not apply the GHS
The option of taking no action was rejected. Maintaining the current classification and information regime for workplace hazardous chemicals in Canada would increasingly place Canadian companies at a disadvantage given that the vast majority of Canada's trading partners (e.g. the United States, the European Union, Australia, Japan, South Korea, China) have applied, or are in the process of applying, the GHS to their workplace hazardous chemicals programs. Maintaining the status quo would also mean that Canadian industry would not benefit from reduced costs that are estimated to be realized by year 4 of the implementation. Canadian industry would also not benefit from the substantial future savings that would result from factors such as not having to reclassify and prepare different labels and safety data sheets (SDSs) for workplace hazardous chemicals being imported from, or exported to, countries that have adopted the GHS approach. This option would also mean that Canadian employers and Canadian workers would fail to benefit from the health and safety advantages afforded by an improved, globally recognized standard for classifying workplace hazardous chemicals and communicating health and safety information to better encourage their safe use.
Option 2. Apply the GHS with no variances with the United States
The option of ensuring absolutely no variances with the United States other than the basic legal requirements (e.g. requiring labels and SDSs to be provided in both official languages) was also rejected as it would have been impossible to do so without reducing current protections for Canadian workers or undermining the established framework of criminal law in Canada for workplace hazardous chemicals. For example, the proposed regulations maintain a Biohazardous Infectious Materials hazard class. This is present in the current Controlled Products Regulations (CPR), but biohazardous infectious materials are not regulated in the U.S. Hazard Communication Standard 2012. Harmonization in this area would have resulted in a reduction in worker protection.
In addition, Canadian law does not provide the latitude to require the classification of a product without setting out clear and objective criteria or defined results. For example, under Canadian law, combustible dusts must be clearly defined in the regulations with objective criteria, whereas OSHA has the ability to set out the criteria in guidance documents.
Option 3. Apply GHS without aligning with the U.S. approach
The GHS sets out standards for classifying hazardous chemicals and communicating hazard information, while recognizing that its application may vary from country to country given the legal and other frameworks of individual countries. Therefore, consideration was also given to simply applying the GHS to Canada's workplace hazardous chemicals program without taking the additional step of aligning to the extent possible with the regulatory approach taken in the United States. While this is achievable from a regulatory perspective, it would fail to take advantage of the opportunity to work with Canada's major trading partner to align further in an area that already has many points of similarity. As a large portion of the workplace hazardous chemicals used in Canada come from sources in the United States and are also exported from Canada into the United States, ensuring as much alignment as possible in terms of the manner in which chemicals are classified and hazards are communicated would not only result in significant savings for industry, but would also provide additional assurances that consistent and clear information would be provided to Canadian employers and workers.
Option 4. Apply GHS in alignment with the United States to the degree possible within Canada's legal and health and safety frameworks (recommended option)
Given the above, implementing the GHS in alignment with the approach taken in the United States while ensuring no loss of current protections in Canada has been retained as the preferred option.
Benefits and costs
A cost-benefit analysis (CBA) was undertaken to assess the impacts of these proposed GHS regulatory changes on key stakeholders, including provincial and territorial governments, industry, employers and workers. (see footnote 10) Within the CBA, a macroeconomic model was developed using data from Industry Canada and Statistics Canada so as to model the number of establishments and employment levels in the various sectors impacted by these changes. In addition, the CBA used information from the U.S. Data and Analysis in Support of an Economic Analysis of Proposed Changes to OSHA Hazard Communication Standard to estimate the number of affected workplace hazardous chemicals by industrial sector and by business size category. In general, the Canadian model is more conservative than that of the United States. The full cost-benefit analysis, Cost Benefit Analysis to Assess the Impacts of Proposed Revisions to Schedule II to the Hazardous Products Act and Amendments to the Controlled Products Regulations — With Revisions to Reflect Updated U.S. Analyses, is available upon request. In addition, the impacts were further assessed through consultations with stakeholders, including a survey of small businesses that was facilitated by several industry associations.
| (Millions of CAN$) | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | 20-Year Total Present Value (PV) (see footnote 11) | Annualized Value |
|---|---|---|---|---|---|---|---|
| A. Cost-benefit analysis (monetized — in CAN$, 2013 price level / constant dollars) Benefits |
|||||||
| Benefits to industry — Productivity | $0.0 | $0.0 | $0.0 | $60.2 | $60.2 | $470.5 | $47.9 |
| Benefits to industry — Updates | $0.0 | $0.0 | $0.0 | $0.0 | $0.0 | $21.4 | $2.2 |
| Benefits to industry — Health and safety | $0.0 | $0.0 | $0.0 | $25.0 | $25.0 | $195.5 | $19.9 |
| Total benefits to industry | $0.0 | $0.0 | $0.0 | $85.2 | $85.2 | $687.5 | $70.0 |
| Costs | |||||||
| Costs to industry — Classification | $22.7 | $22.7 | $0.0 | $0.0 | $0.0 | $43.7 | $4.4 |
| Costs to industry — Training | $111.5 | $111.5 | $0.0 | $0.0 | $0.0 | $214.7 | $21.9 |
| Costs to industry — Printing | $0.0 | $0.0 | $3.1 | $3.1 | $3.1 | $27.1 | $2.8 |
| Total costs to industry | $134.2 | $134.2 | $3.1 | $3.1 | $3.1 | $285.5 | $29.1 |
| Cost to federal government | $1.5 | $1.5 | $0.0 | $0.0 | $0.0 | $2.8 | $0.3 |
| Cost to provincial and territorial governments | $3.9 | $3.9 | $0.0 | $0.0 | $0.0 | $7.6 | $0.7 |
| Total costs to governments | $5.4 | $5.4 | $0.0 | $0.0 | $0.0 | $10.4 | $1.0 |
| Total costs | $139.6 | $139.6 | $3.1 | $3.1 | $3.1 | $295.8 | $30.1 |
| Net Benefits | |||||||
| Net benefits to industry | -$134.2 | -$134.2 | -$3.1 | $82.1 | $82.1 | $402.0 | $40.9 |
| Net benefits to federal, provincial and territorial government | -$5.4 | -$5.4 | $0.0 | $0.0 | $0.0 | -$10.4 | -$1.0 |
| Total net benefits | -$139.6 | -$139.6 | -$3.1 | $82.1 | $82.1 | $391.6 | $39.9 |
| B. Cost-benefit analysis (unquantified) | |||||||
|
|||||||
Assumptions
- The analysis is based on a 20-year time horizon. However, all classification and label/SDS preparation costs would be incurred within two years.
- The costs and benefits have been discounted at 8%. Applying a 7% discount rate would increase discounted costs to small establishments by about 2% under the initial scenario.
- The costs presented here are based on a complex economic model. The model provides estimates of costs based on the expected number of affected substances/mixtures and the number of establishments and establishment size, and estimates of the incremental personnel and information technology/software costs per substance/mixture. Some of the key data sources and assumptions used are as follows:
- the number of establishments in Canada is based on Government of Canada data and is similar to the number found in the Cost Benefit Analysis to Assess the Impacts of Proposed Revisions to Schedule II to the Hazardous Products Act and Amendments to the Controlled Products Regulations — With Revisions to Reflect Updated U.S. Analyses;
- the numbers of affected substances/mixtures per establishment are similar to those applied in the economic analysis for OSHA's Hazard Communication Standard Final Rule as published in the U.S. Federal Register on March 26, 2012, and these vary by sector and by establishment size;
- the personnel costs of classification and label/SDS preparation range from $180 to $315 per affected substance/ mixture depending on the establishment size, with an average of $256, and these are based on inputs to the economic analysis for OSHA's Hazard Communication Standard Final Rule;
- the information technology and software costs for classification and labelling range from about $23 to $214 per SDS depending on the establishment size, with an average of $50, and these are based on inputs to the economic analysis for OSHA's Hazard Communication Standard Final Rule; and
- it is assumed that the number of substances/mixtures that have already been classified and had GHS-compliant labels and SDSs prepared is between 1% and 50%, depending on the establishment size.
Benefits
Productivity benefits for storage, distribution and use of workplace hazardous chemicals ($470.5M — 20-year PV)
Due to the current non-uniformity of SDSs and labels, managers and other staff devote time to determining the right course of action related to storage, handling, and use of workplace hazardous chemicals. This is often done on a daily basis. The GHS standard, by making the structure and layout of SDSs uniform, would help to reduce the time required to manage workplace hazardous chemicals. The consistency of the information to be provided on labels and in the new SDSs would make it more efficient to prepare in-plant labels, determine storage requirements, and understand the chemical's uses and precautions that must be taken.
These benefits were estimated by considering time savings for health and safety managers, health and safety supervisors, and logistics staff in the goods-producing sectors that would result from the implementation of the GHS. These time savings figures, based on those employed in the U.S. model, were estimated as 1.5%, 0.75%, or 7.5% for these three groups, respectively.
Using these estimates of time savings from the GHS, as well as the number of staff, annual hours worked, and the relevant wage, the estimated total annual productivity benefits are $60.2 million. Over a 20-year period, it is estimated that the PV of the productivity benefits would be $470.5 million. These benefits represent only 8.6% of the $698.6 million annual productivity benefits estimated for the United States, as again, Canada used a more conservative model than that of the United States.
Cost savings for updates of SDSs and labels of workplace hazardous chemicals ($21.4M — 20-year PV)
Firms review products periodically by gathering physical and health hazard data for ingredients of workplace hazardous chemicals, reviewing the classification calculations, and modifying associated SDSs and labels. The standardized GHS system for classification and the consistency of the information to be provided would result in time savings when companies undertake future updates of SDSs and labels.
These benefits were estimated by calculating the number of annual updates and assuming a savings for each update. Based on the U.S model, the figure of 25% was assumed in the Canadian model. It is estimated that it takes companies between four and seven hours to produce an SDS.
Using the estimate of a 25% time savings, as well as the number of SDSs/labels per establishment in Canada and how often they need to be updated, the average time per update, and wages, annual benefits of $3.4 million are anticipated, or 7% of the $47.3 million in annual benefits expected in the United States. Over a 20-year period, it is estimated that the PV of the cost-savings for updates of SDSs and labels would be $21.4 million.
Health and safety benefits ($195.5M — 20-year PV)
The adoption of the GHS for workplace hazardous chemicals in Canada would be expected to result in health and safety benefits for Canadian workers, including fewer personal injuries, fewer acute and chronic illnesses, and fewer fatalities. Baseline data for the number of injuries, illnesses, and fatalities attributable to workplace hazardous chemicals is not currently available in Canada. Therefore, estimates of the negative health impacts of workplace hazardous chemicals in Canada, and the reduction in these impacts expected to result from the GHS, have been extrapolated from analyses done in the United States.
Previous workplace health and chemicals analyses completed in the United States estimated the health benefits resulting from the country's original workplace hazard program. The OSHA estimated that health benefits resulting from the adoption of the GHS could be equal to roughly 1% of the value of the health benefits from the original workplace hazard program. Based on this model, it is estimated that the adoption of the GHS in Canada would result in the annual prevention of 30 non-lost-workday injuries and illnesses, 20 lost-workday injuries and illnesses, 6 chronic illnesses, and 4 fatalities in Canada. The socio-economic value of these projected health impacts was estimated to be $30.5 million per year. Over a 20-year period, it is expected that the PV of health and safety benefits would be $195.5 million.
Benefits to the economy, business and trade (unquantified)
In addition to the aforementioned quantified benefits, Canadian businesses would gain substantial unquantified benefits once this new regime is fully in place.
First, they would garner significant benefit from not having to reclassify and prepare new labels and SDSs for workplace hazardous chemicals imported from other countries or when exporting to other countries that also use the GHS regime. Currently, when workplace hazardous chemicals are imported into Canada (or exported from Canada to other countries including the United States and the European Union), suppliers need to reclassify the chemicals as well as label and prepare SDSs according to the hazard communications system in place in the specific country (or countries) in which they are selling their products. Once the GHS is implemented in Canada, suppliers would no longer have to do this for the vast majority of chemicals being imported from or exported to countries that have adopted the GHS. While information on imports and exports was not available to quantify this impact, it is recognized by industry that this change has the potential to deliver significant savings to Canadian businesses.
Second, suppliers and employers would see economic benefits related to the fact that it would be easier and faster to train workers in the future, and this training would be more effective. The hazard communications elements of the GHS are more easily understood (due to the standardization of language and more comprehensible pictograms in certain cases), and generic training materials would be available. These benefits are incremental to the other safety and health related benefits identified above. Finally, again while not quantifiable, it is clear that adopting this regime in Canada in alignment with the United States would have significant trade benefits for the sector as barriers are reduced as a result of common labelling and other information requirements.
Costs
Costs to government ($10.4M — 20-year PV)
Based on the cost-benefit analysis, it is expected that the Government of Canada would face $3 million in regulatory and program costs in the first two years of implementation. Specifically, the funds will be dedicated to regulatory oversight, compliance promotion and outreach, and enforcement activities. This same analysis also estimated that each provincial and territorial (P/T) government would incur an average of $300,000 in costs in the first two years of implementation (for a two-year P/T total of $7.8 million) resulting from the need to revise and adjust OSH regulations and programs.
Classification ($43.7M — 20-year PV)
Core costs of implementing GHS would be those related to the reclassification of products according to the GHS and the subsequent preparation of GHS-compliant SDSs and labels. These costs include both personnel costs and software/IT costs.
The costs within this category are based solely on the cost of reclassifying existing chemicals based on the new classification criteria, as it is assumed that future costs associated with new chemicals are the same or comparable to those under the current WHMIS framework.
The first cost component related to reclassification and SDS and label revision is personnel costs. A team of toxicologists, industrial hygienists, SDS writers, and computer programmers are expected to conduct the task of reclassifying chemicals and modifying SDS and labels. These professionals would have to gather the existing data on the hazards and other characteristics of their chemicals, apply the GHS criteria to determine the hazard classes and categories, establish a uniform system for revising existing SDSs and labels, and make the necessary revisions.
To generate these estimates, it was assumed that it takes Canadian businesses between four and seven hours per SDS, depending on the establishment size. This assumption was based on the U.S model. It was then calculated that some products would already have been classified based on the GHS standard (in particular, where products are exported or imported). GHS-compliant products were calculated at a rate of 1% for companies of 1–4 employees, 5% for companies of 5–99 employees, 25% for companies with 100–499 employees and 50% for companies with more than 500 employees. With data from Industry Canada, it was estimated that approximately 150 000 products would be affected by the adoption of the GHS in Canada. Using these figures, as well as the wage rate, it was estimated that personnel costs would be $38.0 million. This includes the cost estimate of classification (50% of the cost or $19 million) as well as the cost of using the information gathered during the initial steps of reclassification to develop a GHS-compliant SDS (25% of the cost or $9.5 million) and a GHS-compliant label (25% of the cost or $9.5 million).
The second cost component related to reclassification and SDS and label revision is software/IT costs. These costs vary depending on the number of SDSs produced by a company. For example, if a company produces only a few SDSs and labels, it could use a standard word-processing program. If the company produces many SDSs, then it may use relational databases or proprietary software.
An approach similar to the one used in the United States was used to monetize costs. Under this approach, costs would range from $23/SDS for a small establishment (fewer than 100 employees) to $225/SDS for large establishments (more than 100 employees). Furthermore, 50% of establishments with 100–499 employees and 95% of establishments with more than 500 employees would upgrade their software. This approach yields Canadian software and IT cost estimates of $7.4 million.
Training ($214.7M — 20-year PV)
Two sets of training costs are relevant with respect to implementing the GHS: the costs of training production workers and the costs of training other key staff. The costs below reflect the additional costs that would be carried to train staff on the new GHS over the first two years of implementation.
Training production workers
The costs of training production workers are the sum of the costs of production worker (trainee) training time and trainer time devoted to training. To estimate the costs, the number of workers in the affected sectors was multiplied by the percentage of production workers who would require GHS training. It was estimated that there would be one hour of training and that the average worker wage rate was $25/hour. The total worker time costs were calculated using data from Industry Canada on the total number of workers and the percentage of these who are production workers, as well as the training duration and the worker wage rate. This approach yields estimated worker training costs of $121.6 million.
The costs of trainer time were estimated based on the time costs of trainers delivering the training (training duration of one hour and trainer wage rate of $45/hour). The number of workers to be trained and the number of workers present at each training session were also taken into account in the calculations. The total trainer time costs were estimated to be $21.9 million.
The sum of worker and trainer costs for GHS training is estimated to be $143.5 million. Worker time costs account for 85% ($121.6 million) of this total and trainer time for 15% ($21.9 million).
Training for other key staff
Three other groups besides production workers would require GHS training: corporate health and safety managers (those who oversee corporate health and safety), health and safety supervisors (those who manage health and safety programs), and logistics staff (those engaged in logistics operations in manufacturing plants, such as receiving, distributing and handling workplace hazardous chemicals on a daily basis). For goods-producing sectors, it was estimated that there would be 0.5 health and safety managers for companies with 100–499 employees and one health and safety manager for businesses with more than 500 employees. It was also estimated that health and safety supervisors represent 2.5% of employees in the model and logistics personnel 0.33% of employees. These estimates are based on those in the U.S. model.
For the service-producing sectors, the same estimates were used for health and safety managers, with an additional 2.5% of employees in this category and no logistics staff. These estimates yield Canadian estimates of staff in each position that are about 10% of the U.S. numbers.
Health and safety managers
The model estimated that there are approximately 300 000 health and safety managers who would require training. The costs for training health and safety managers were calculated using the number of managers (calculated as described above), the costs of a course, and time for managers. For example, in the manufacturing sector, it is assumed that a health and safety manager would take an eight-hour course at a cost of $800 and a time cost of $400. In the non-manufacturing and services sectors, it is assumed that a shorter course (for example online) would be taken through self-training. This approach yields training costs of $49.2 million.
Health and safety supervisors
Based on calculations described above, it was estimated that there are approximately 75 000 health and safety supervisors in Canada (including full- and part-time workers). It is assumed that the supervisors in the goods-producing sectors self-train for eight hours at a wage rate of $45 per hour. It is therefore estimated that training these employees would cost $27.0 million.
Logistics staff
The Canadian model shows approximately 10 000 logistics staff, all within the goods-producing sectors. The costs for training these staff were estimated at eight hours of self-training at a staff wage of $40 per hour. Based on these numbers, logistics staff training costs of about $3.2 million are anticipated.
The total training costs for training other key staff (health and safety managers, health and safety supervisors, and logistics staff) are estimated to be $79.4 million:
- $49.2 million for approximately 300 000 managers;
- $27.0 million for approximately 75 000 supervisors; and
- $3.2 million for approximately 10 000 logistics staff.
As expected, the manufacturing sector would face the most significant costs for training other key staff, totalling about $17 million. All other sectors would face training costs below $10 million.
Total training costs
The total training costs are the sum of costs for training production workers (including trainee and trainer costs) and the costs of training others (including health and safety managers and supervisors, and logistics staff). These costs were estimated in the two previous sections and total $222.9 million, with production worker training costing $143.5 million and other staff training costing $79.4 million.
| Sector | Staff Group | Training Cost |
|---|---|---|
| Production workers | Production workers | $121.6 million |
| Trainers | $21.9 million | |
| Total for production workers | $143.5 million | |
| Other key staff | Health and safety managers | $49.2 million |
| Health and safety supervisors | $27.0 million | |
| Logistics staff | $3.2 million | |
| Total for other key staff | $79.4 million | |
| TOTAL TRAINING COSTS | $222.9 million | |
These training costs would be split between the first two years of implementation. The 20-year PV of these costs would be $214.7 million.
Printing ($27.1M — 20-year PV)
While the current labels are black and white, the labels proposed in the regulations require colour. Some businesses would incur initial costs to purchase upgraded SDS and label printing equipment and supplies or to purchase preprinted color labels. In order to determine costs, businesses were categorized as companies currently printing in black and white and having no colour printer, companies currently printing in black and white but already owning a colour printer, companies using preprinted materials and companies already printing in colour. Costs were determined by estimating incremental costs of upgrading printers (and the number of printers that would be required) and the costs of additional or upgraded cartridges, ribbons and printing stock. The results of the modelling suggest an incremental annual colour printing cost of $3.1 million. The 20-year PV of the costs is $27.1 million.
Summary of estimated costs to industry
The total costs to industry are estimated to be one-time costs of $268.3 million ($45.4 million in classification, SDS development, and label development costs, $143.5 million in production worker training costs, and $79.4 million in other staff training costs) and incremental costs of $3.1 million per year (for colour printing).
Summary of costs and savings for industry
The following assumptions were made regarding the timing of costs and benefits expected to be realized through the adoption of the GHS for workplace hazardous chemicals:
Costs
- companies would spend an estimated $22.7 million annually for classifying workplace hazardous chemicals and preparing GHS-compliant labels and SDSs in 2014 and 2015;
- companies would spend an estimated $111.5 million annually for training production workers and others (health and safety and logistics staff) in 2014 and 2015; and
- companies would spend an estimated $3.1 million annually in incremental colour printing beginning in 2016.
Savings
- annual cost savings estimated at $3.4 million from easier and faster updates of SDSs and labels are realized in 2019 and all future years (i.e. no benefits occur until three years after implementation);
- overall annual savings (due to productivity and health and safety benefits) for the industry are estimated at $82.1 million commencing in year 2017, which equates to $402.0 million over a 20-year period (based solely on quantifiable benefits); and
- further significant savings from not having to reclassify and provide new labels and SDSs for different markets, from easier and more effective training in the future and from the reduction in trade barriers are anticipated (not quantified).
The cost-benefit analysis contains several scenarios that assess the sensitivity of the results to key input assumptions, with the following results:
- doubling the number of affected workplace chemicals has a relatively small effect, and lowers the net present value (NPV) of benefits to $369.3 million;
- halving the assumed productivity gains — the major quantifiable driver of benefits — greatly diminishes the NPV of benefits to $234.8 million; and
- increasing the social discount rate from 8% to 12% reduces the NPV of benefits to $213.8 million.
“One-for-One” Rule
The “One-for-One” Rule does not apply to this regulatory proposal as the proposal does not contain requirements that would place an administrative burden on industry.
The proposed Hazardous Products Regulations contain requirements for classifying workplace hazardous chemicals and for communicating related information to the purchasers of these products on product labels and safety data sheets. The costs associated with classifying, labelling and providing safety data sheets to purchasers are “compliance costs” as defined in the Guide for the “One-for-One” Rule. While there would be some short-term compliance costs for industry as part of the transition to the new system, over the medium term to long term, substantial benefits would result from both an improved trade environment and lower compliance costs (in particular, from a substantial reduction in the need to reclassify and relabel workplace hazardous chemicals for different markets).
The proposal does not contain provisions that require industry to demonstrate compliance with the regulations, such as collecting, processing, reporting and retaining information, or completing forms or other paperwork, which are considered “administrative costs” by the Treasury Board Secretariat. The “One-for-One” Rule relates only to “administrative costs”; therefore, it does not apply to this regulatory proposal. Moreover, the adoption of this proposal would coincide with the repeal of existing outdated regulations, so there would be a reduction in the number of regulations to which industry is subject.
The consequential amendments, including those made under the Canadian Environmental Protection Act, 1999 (New Substances Notification Regulations (Chemicals and Polymers) and Export of Substances on the Export Control List Regulations), as well as the amendments to the Hazardous Materials Information Review Regulations and Hazardous Materials Information Review Act Appeal Board Procedures Regulations resulting from recent changes to the Hazardous Materials Information Review Act, ensure that the definitions and terminology in these regulations reflect those in the proposed Hazardous Products Regulations and the amended Hazardous Materials Information Review Act, respectively. No changes would be made to the regulatory process of claiming an exemption to protect confidential business information. Therefore, there would be no associated administrative costs.
Small business lens
The proposed Hazardous Products Regulations would result in a net reduction in costs faced by small businesses. The small business lens does not apply.
The cost-benefit analysis described above shows that, overall, businesses will face a reduction in costs as a result of the implementation of the GHS. Small businesses would face one-time compliance costs associated with adapting to the new system similar to the types of costs faced by larger businesses. However, after the first two years, small businesses would see substantial savings that would continue to accrue. For example, based on the cost-benefit analysis done in the United States, the Canadian model assumed that the implementation of the GHS would result in 25% time savings for businesses with fewer than 100 employees. It would be faster and easier to update the GHS-compliant labels and SDSs, and small businesses would see their costs decrease as a result.
This statement was confirmed by a survey of small businesses conducted by Health Canada in 2013. The responses consistently supported the implementation of the GHS because of the medium- and long-term cost savings for small businesses. For example, one small business identified that it would see financial benefit in not having to reclassify substances being imported from the United States. Similarly, another respondent indicated that their small business would see savings of $20,000 in relabelling costs alone. While those responding to the survey acknowledged that there would be upfront costs associated with the GHS implementation, they indicated that those costs were far outweighed by the long-term benefits.
The consequential amendments to other sets of regulations and the changes to the Hazardous Materials Information Review Regulations and Hazardous Materials Information Review Act Appeal Board Procedures Regulations would have no effect on the costs faced by small businesses.
Consultation
Canadian stakeholders (i.e. representatives of federal, provincial and territorial governments, suppliers, employers and workers) have been involved in the development of the GHS, under the auspices of the United Nations, for more than 20 years.
Over the years, Health Canada has consulted at length with stakeholders on the GHS and its implementation regarding workplace hazardous chemicals. This has been done under the Intergovernmental WHMIS Coordinating Committee, which is made up of representatives of federal, provincial and territorial occupational health and safety (OSH) agencies, as well as with the WHMIS Current Issues Committee, which is made up of Intergovernmental WHMIS Coordinating Committee members as well as representatives from supplier, employer, and worker organizations.
Over the past three years in particular, a series of stakeholder meetings has taken place to discuss the regulatory proposals and to better understand the context in the United States, as well as internationally.
From June 29, 2013, to September 15, 2013, Health Canada held a public consultation on an early version of the regulatory proposal. Written submissions were received from industry associations, suppliers, employers, provincial and territorial governments, worker organizations, health groups, occupational health and safety professionals and individuals. In addition, the proposal and related comments were discussed with stakeholders at face-to-face meetings in Ottawa in October 2013. These comments and discussions were taken into consideration in arriving at the current regulatory proposal.
Provinces and territories are supportive of the implementation of the GHS and have been key partners in the development of the regulatory framework. Their primary concern is ensuring current worker protections are not reduced through regulatory alignment. Taking into consideration that amendments to federal, provincial and territorial OSH legislation and regulations will be required, they also want to ensure that they will have adequate time to transition to the GHS and that there is consistency across the country throughout the transition.
Suppliers of workplace hazardous chemicals are strong supporters of alignment with the United States, and with the GHS more broadly. Some supplier associations advocated for the inclusion of the GHS in the RCC Action Plan. Both small and large companies have recognized that the one-time costs associated with implementing the new system are far outweighed by the ongoing savings that result in the long term from having the same classification and hazard communication system as that of the United States. Their primary concern is with regard to the timing of implementation. They would like Canada and the United States to align their implementation timelines to the greatest extent possible in order to minimize the amount of time needed for two separate classification and hazard communication systems to be in place.
Suppliers are also anxious to ensure that regulatory variances between Canada and the United States are kept to an absolute minimum. The number of regulatory variances has decreased through regular dialogue and amended regulatory proposals, and the variances that remain are necessary in order to either maintain the current level of protections afforded to Canadian workers or to respect the framework of the Canadian legislation and regulations. Nevertheless, suppliers have remaining concerns on some of the proposed variances due to the potential impact of less-than-complete alignment with the United States. Some suppliers would also like to see greater alignment with other jurisdictions, such as the European Union.
Employers and worker organizations also support the implementation of the GHS and want to ensure that worker health and safety protections are maintained or expanded (and not reduced) through alignment with the United States. Employers would face additional training costs associated with the implementation of the GHS, but are likely to see accrued benefits as a result of workers receiving clearer and more consistent hazard communication information and, consequently, fewer workplace injuries and illnesses.
Health Canada continues to engage key partners and stakeholders through regular teleconferences and meetings, presentations at association events, and ongoing one-on-one dialogue.
These regulatory proposals are designed to continue the regulatory cooperation upon which WHMIS is based. The regulatory regime under the Hazardous Products Act will continue to establish the requirements related to the classification and labelling of workplace hazardous chemicals by suppliers, and federal, provincial, and territorial OSH legislation and regulations will continue to establish the employer requirements for information and training at the workplace level. Therefore, collaborative work has been, and would continue to be, undertaken with federal, provincial and territorial governments to coordinate these two key components of Canada's workplace hazardous chemicals program.
Rationale
Applying the GHS to Canada's hazard communication system for workplace hazardous chemicals, and doing so in alignment with the approach being taken by the United States, Canada's major trading partner, would not only reduce costs for industry and facilitate trade, but also keep Canada on pace with the global standard for such systems. The benefits are clear:
- Canadian industry would start seeing benefits ($82 million) as soon as the fourth year of implementation. The net benefits for the Canadian government and industry would be $391.6 million (PV) over the next 20 years.
- Workplaces in Canada would be safer and hundreds of potential workplace injuries would be prevented.
- The harmonization of the Canadian workplace hazardous chemicals classification system with those of virtually all of our trading partners, including the United States, would facilitate trade.
- Maintaining the current regulatory approach would mean that Canada's workplace hazardous chemicals regime would be increasingly out of step with not only the approach being adopted in the United States, but also the approach being taken by the vast majority of Canada's trading partners. Trade barriers would increase, as would administrative costs for Canadian industries. This would cost these industries millions in lost revenue.
- The training of workers would be more efficient and effective, bringing increased health and safety benefits.
Health Canada has consulted widely with key partners and stakeholders, who have expressed support for the proposed approach. Industry is particularly supportive of this work, and has been encouraging timely action along these lines.
Other options have been considered, and analysis has determined that implementation of the GHS in alignment with the U.S. approach to the maximum extent possible is the best way to proceed. The proposed regulations would reduce costs for industry, facilitate trade, maintain worker protections, and keep Canada on pace with the global standard for such systems.
Implementation, enforcement and service standards
Implementation
The intention is to move forward with these proposed regulatory changes in a timely manner in order to have the amended regulations in force on or before June 1, 2015, to align with the date upon which the use of GHS labels and safety data sheets (SDSs) becomes mandatory for manufacturers and importers in the United States.
Health Canada is working to finalize the regulations by December 1, 2014. A transition period would begin when the regulations come into force, on or before June 1, 2015. Similar to the transition to the GHS in the United States, the transition to the GHS in Canada would take place in phases, giving key partners and stakeholders sufficient time to make the necessary regulatory and system adjustments. Attention would also be given to ensuring consistency across Canada through coordination and alignment between federal, provincial, and territorial jurisdictions.
As has been the approach to date, the intent would be to continue to work with federal, provincial and territorial partners, as well as other stakeholders, to assist them in developing the necessary information and training materials. In addition, in consultation with these partners and stakeholders, Health Canada would develop core information materials, key guidance documents and training materials to ensure the proposed changes are fully understood. The guidance material would be made public when the regulations are finalized. The key mechanisms for continuing this needed collaboration would be the two core WHMIS committees: the Intergovernmental WHMIS Coordinating Committee and the WHMIS Current Issues Committee. In addition, work would continue with the Canadian Association of Administrators of Labour Legislation — Occupational Safety and Health (OSH) Committee to help ensure the implications of the proposed changes to the Controlled Products Regulations for OSH legislation and regulations are understood, and to help coordinate the approach to the management of transition issues and final implementation.
Enforcement
Health Canada would continue to undertake inspection activities as provided for under the Hazardous Products Act (HPA), including the practice of designating qualified individuals at federal, provincial, and territorial OSH agencies as HPA inspectors. Working with the Intergovernmental WHMIS Coordinating Committee and the Canadian Association of Administrators of Labour Legislation — Occupational Safety and Health Committee, Health Canada would develop training materials and undertake training sessions for federal, provincial and territorial OSH inspectors to promote a common understanding of the new regulations.
Contact
Amira Sultan
Workplace Hazardous Materials Directorate
Department of Health
Postal Locator 4707A
427 Laurier Avenue West, 7th Floor
Ottawa, Ontario
K1A 0K9
Telephone: 1-855-407-2665
Fax: 613-993-5016
Email: whmis_simdut@hc-sc.gc.ca
PROPOSED REGULATORY TEXT
Notice is given that the Governor in Council, pursuant to subsection 15(1) (see footnote a) of the Hazardous Products Act (see footnote b), proposes to make the annexed Hazardous Products Regulations.
Interested persons may make representations concerning the proposed Regulations within 30 days after the date of publication of this notice. All such representations must cite the Canada Gazette, Part I, and the date of publication of this notice, and be addressed to Amira Sultan, Workplace Hazardous Materials Directorate, Department of Health, Postal Locator: 4707A, 427 Laurier Avenue West, 7th Floor, Ottawa, Ontario K1A 0K9 (tel.: 1-855-407-2665; fax: 613-993-5016; email: whmis_simdut@hc-sc.gc.ca).
Ottawa, July 31, 2014
JURICA ČAPKUN
Assistant Clerk of the Privy Council
HAZARDOUS PRODUCTS REGULATIONS
PART 1
INTERPRETATION
Definitions
1. (1) The following definitions apply in these Regulations.
“Act”
« Loi »
“Act” means the Hazardous Products Act.
“aerosol dispenser”
« générateur d'aérosol »
“aerosol dispenser” means a receptacle made of metal, glass or plastic and containing a gas that is compressed, liquefied or dissolved under pressure, with or without a liquid, paste or powder, and fitted with a release device allowing the contents to be ejected in the form of solid or liquid particles in suspension in a gas, as a foam, a paste or a powder or in a liquid or gaseous state.
“ATE”
« ETA »
“ATE” means an acute toxicity estimate, and includes the LD50 and the LC50, and the acute toxicity point estimate determined in accordance with the table to section 8.1.7.
“CAS registry number”
« numéro d'enregistrement CAS »
“CAS registry number” means the identification number assigned to a chemical by the Chemical Abstracts Service, a division of the American Chemical Society.
“chemical name”
« dénomination chimique »
“chemical name” means a scientific designation of a material or substance that is made in accordance with the rules of nomenclature of either the Chemical Abstracts Service, a division of the American Chemical Society, or the International Union of Pure and Applied Chemistry, or a scientific designation of a material or substance that is internationally recognized and that clearly identifies the material or substance.
“flash point”
« point d'éclair »
“flash point” means the lowest temperature, corrected to the standard pressure of 101.3 kPa, at which the application of an ignition source causes the vapours of a liquid to ignite.
“gas”
« gaz »
“gas” means a mixture or substance that
- (a) at 50°C has an absolute vapour pressure of greater than 300 kPa; or
- (b) is completely gaseous at 20°C and at the standard pressure of 101.3 kPa.
“GHS”
« SGH »
“GHS” means the United Nations document entitled Globally Harmonized System of Classification and Labelling of Chemicals (GHS), Fifth Revised Edition.
“hazardous ingredient”
« ingrédient dangereux »
“hazardous ingredient” means an ingredient in a mixture that, when evaluated as an individual substance, is classified in a category or subcategory of a health hazard class.
“hazard statement”
« mention de danger »
“hazard statement” means a phrase assigned to a category or subcategory of a hazard class or, in the case of column 5 of Parts 4 to 6 of Schedule 5, the required statement that describes the nature of the hazard presented by a hazardous product.
“initial boiling point”
« point d'ébullition initial »
“initial boiling point” means the temperature of a liquid at which its vapour pressure is equal to the standard pressure of 101.3 kPa, i.e., the temperature at which the first gas bubble appears.
“initial supplier identifier”
« identificateur du fournisseur initial »
“initial supplier identifier” means the name, address and telephone number of
- (a) the manufacturer; or
- (b) the importer of the hazardous product who operates in Canada.
“LC50”
« CL50 »
“LC50” means the concentration of a mixture or substance in air that causes the death of 50.0% of a group of test animals.
“LD50”
« DL50 »
“LD50” means the single dose of a mixture or substance that, when administered by a particular exposure route in an animal study, is expected to cause the death of 50.0% of a given animal population.
“liquid”
« liquide »
“liquid” means a mixture or substance that
- (a) at 50°C has a vapour pressure of 300 kPa or less;
- (b) is not completely gaseous at 20°C and at the standard pressure of 101.3 kPa; and
- (c) has a melting point or initial melting point of 20°C or less at the standard pressure of 101.3 kPa or, in the case of a mixture or substance for which neither can be determined, is shown
- (i) to be a liquid as a result of the ASTM International method ASTM D4359-90, entitled Standard Test Method for Determining Whether a Material Is a Liquid or a Solid, as amended from time to time, or
- (ii) to not be pasty as a result of the test for determining fluidity (penetrometer test), referred to in section 4 of chapter 3 of Part 2, numbered 2.3.4, of Annex A of the European Agreement Concerning the International Carriage of Dangerous Goods by Road, as amended from time to time.
“Manual of Tests and Criteria”
« Manuel d'épreuves et de critères »
“Manual of Tests and Criteria” means the United Nations document entitled Recommendations on the Transport of Dangerous Goods: Manual of Tests and Criteria, as amended from time to time.
“manufacturer”
« fabricant »
“manufacturer” means a supplier who, in the course of business in Canada, manufactures, produces, processes, packages or labels a hazardous product and sells it.
“OECD”
« OCDE »
“OECD” means the Organisation for Economic Co-operation and Development.
“outer container”
« contenant externe »
“outer container” means the most outward container of a hazardous product that is visible under normal conditions of handling, but does not include the most outward container if it is the only container of the hazardous product.
“pictogram”
« pictogramme »
“pictogram” means a graphical composition that includes a symbol along with other graphical elements, such as a border or background colour.
“precautionary statement”
« conseil de prudence »
“precautionary statement” means a phrase that describes the recommended measures to take in order to minimize or prevent adverse effects resulting from exposure to a hazardous product or resulting from improper storage or handling of a hazardous product.
“product identifier”
« identificateur de produit »
“product identifier” means, in respect of a hazardous product, the brand name, chemical name, common name, generic name or trade name.
“risk group classification”
« classification par groupe de risque »
“risk group classification” means, in relation to the “Biohazardous Infectious Materials” health hazard class, classification in Risk Group 2, Risk Group 3 or Risk Group 4 as defined in subsection 3(1) of the Human Pathogens and Toxins Act.
“SADT” or “self- accelerating decomposition temperature”
« TDAA » ou « température de décomposition autoaccélérée »
“SADT” or “self-accelerating decomposition temperature” means the lowest temperature at which self-accelerating decomposition occurs.
“scientifically validated method”
« méthode validée sur le plan scientifique »
“scientifically validated method” means, in relation to a hazard, a method that specifies standards for the evaluation of that hazard and whose results are accurate and reproducible, in accordance with established scientific principles.
“signal word”
« mention d'avertissement »
“signal word” means, in respect of a hazardous product, the word “Danger” or “Warning” that is used to alert the reader to a potential hazard and to indicate its severity.
“solid”
« solide »
“solid” means a mixture or substance that is not a liquid or gas.
“United Nations Model Regulations”
« Règlement type des Nations Unies »
“United Nations Model Regulations” means the United Nations document entitled Recommendations on the Transport of Dangerous Goods: Model Regulations, as amended from time to time.
“UN number”
« numéro ONU »
“UN number” means the four-digit identification number issued in accordance with the United Nations Model Regulations.
“vapour”
« vapeur »
“vapour” means the gaseous form of a mixture or substance released from its liquid or solid state.
“work place”
« lieu de travail »
“work place” means a place where a person works for remuneration.
Reference to hazard class
(2) In these Regulations, a reference to a hazard class is to be read as a reference to a hazard class that is listed in Schedule 2 to the Act.
Health professionals
(3) For the purposes of Parts 5 and 6, health professionals are
- (a) physicians who are registered and entitled under the laws of a province to practise medicine and who are practising medicine under those laws in that province; and
- (b) nurses who are registered or licensed under the laws of a province to practise nursing and who are practising nursing under those laws in that province.
Interpretation of “should”
(4) When the word “should” is used in a text that is referenced or incorporated in these Regulations, it is to be read as imperative, unless the context requires otherwise.
PART 2
CLASSIFICATION OF A PRODUCT, MIXTURE, MATERIAL OR SUBSTANCE
GENERAL
Order of decreasing severity
2. (1) In each Subpart of Parts 7 and 8, the categories and subcategories in each of the classification tables to those Subparts are set out in the order of the hazard's decreasing severity, except for the categories of the classification table to Subpart 5 of Part 7.
Evaluation — more severe hazard
(2) If a product, mixture, material or substance has been evaluated in accordance with the criteria and requirements of a category or subcategory of a hazard class that represents the more severe hazard in a classification table compared to another category or subcategory of that hazard class in the same classification table and is classified in that category or subcategory, the product, mixture, material or substance need not be evaluated in respect of a category or subcategory of the same classification table of the same hazard class that represents a less severe hazard.
Prescribed classification
(3) Subject to subsections (4) and (5), any product, mixture, material or substance for which classification in a category or subcategory of a hazard class is prescribed in Schedule 4 is classified in that category or subcategory. The product, mixture, material or substance must also be evaluated in accordance with section 2.1, 2.2 or 2.7 in respect of each of the categories or subcategories of the other hazard classes.
Ingredient — more severe hazard
(4) If a product, mixture, material or substance is one for which classification in a category or subcategory of a hazard class is prescribed in Schedule 4, and if it has been mixed with one or more ingredients that are classified in a category or subcategory of the same classification table of the same hazard class that represents a more severe hazard, the mixture as a whole must be classified in the category or subcategory that represents the more severe hazard.
Prescribed classification — Subpart 1, 4, 7 or 8 of Part 8
(5) A mixture, material or substance — for which classification in a category or subcategory of a classification table of a hazard class set out in Subpart 1, 4, 7 or 8 of Part 8 is prescribed in Schedule 4 — must also be evaluated in accordance with section 2.1 or 2.2, in the case of Subparts 1, 4 or 7 of Part 8 in respect of each of the categories or subcategories of the other classification tables of the same hazard class, and in the case of Subpart 8 of Part 8, in respect of each of the categories of the same classification table.
Impurities, stabilizing solvents and stabilizing additives — substance
(6) Any impurities, stabilizing solvents or stabilizing additives that are known to the supplier to be present in a substance and that are classified must be considered for the purpose of classification of the substance if they are present at a concentration above the concentration limit for an ingredient in a mixture set out in a particular category or subcategory of any hazard class.
Impurities, stabilizing solvents and stabilizing additives — mixture
(7) Any impurities, stabilizing solvents or stabilizing additives that are known to the supplier to be present in a mixture and that are classified must be considered for the purpose of classification of the mixture if they are present at a concentration above the concentration limit for an ingredient in a mixture set out in a particular category or subcategory of any hazard class.
Individually packaged in outer container
(8) If two or more different and individually packaged products, mixtures, materials or substances, designed to be accessed individually, are packaged together in an outer container for sale or import, the assemblage of the products, mixtures, materials and substances in the outer container must not be considered as a single product for the purpose of classification, as each product, mixture, material or substance is subject to the classification provisions of this Part.
MATERIAL OR SUBSTANCE
Classification — material or substance
2.1. Subject to sections 2.8 and 2.9, for the purpose of establishing whether a material or substance is classified in a category or subcategory of a hazard class, the material or substance must be evaluated in accordance with established scientific principles, with respect to the criteria and requirements of each category or subcategory of the hazard class as set out in Parts 7 and 8, using available data of the following types, as applicable:
- (a) in relation to the material or substance itself,
- (i) results of testing or studies carried out in accordance with the test methods referred to in Part 7 or 8,
- (ii) results of testing or studies carried out in accordance with generally accepted standards of good scientific practice at the time the test or study was carried out,
- (iii) conclusions based on established scientific principles, and
- (iv) case reports or documented observations; and
- (b) except for Subparts 2 and 3 of Part 8, if the data of the types referred to in paragraph (a) are insufficient to evaluate the material or substance in accordance with the criteria and requirements set out in Parts 7 and 8, in relation to a material or substance that has similar properties,
- (i) results of testing or studies carried out in accordance with the test methods referred to in Part 7 or 8,
- (ii) results of testing or studies carried out in accordance with generally accepted standards of good scientific practice at the time the test or study was carried out,
- (iii) conclusions based on established scientific principles, and
- (iv) case reports or documented observations.
MIXTURE
Classification
Part 7
2.2. (1) Subject to section 2.9, for the purpose of establishing whether a mixture is classified in a category or subcategory of a physical hazard class, the mixture must be evaluated, in respect of each category or subcategory of each physical hazard class, using data of the types referred to in subparagraphs 2.1(a)(i) to (iv) in relation to the mixture or, if the data of those types are insufficient to evaluate the mixture in accordance with the criteria and requirements set out in Part 7, using data of the types referred to in subparagraphs 2.1(b)(i) to (iv) in relation to a mixture with similar properties.
Part 8
(2) Subject to section 2.8, for the purpose of establishing whether a mixture is classified in a category or subcategory of a health hazard class, the mixture must be evaluated, in respect of each category or subcategory of each health hazard class, using data of the types referred to in subparagraphs 2.1(a)(i) to (iv), in relation to the ingredients, the mixture as a whole or in relation to a mixture with similar properties, following the order of the provisions, in relation to mixtures, as presented in each Subpart of Part 8.
Part 8 — order of provisions
(3) When following the order of the provisions in accordance with subsection (2), the mixture must be classified in accordance with the first provision that permits its classification. Once the mixture is classified, the provisions that follow within the same Subpart in relation to mixtures do not apply, except in the case of Subparts 1, 4, 7 and 8 of Part 8.
Bridging Principles
Definitions
2.3. (1) The following definitions apply in this section.
“production batch”
« lot de fabrication »
“production batch” means a batch that results from a consistent production process using fixed physico-chemical parameters when there is no intention to alter the characteristics of the final product.
“tested”
« testé »
“tested” refers to a mixture for which there are data of a type referred to in subparagraph 2.1(a)(i), (ii) or (iv).
Application of bridging principles
(2) In the case of the health hazard classes set out in Subparts 1 to 10 of Part 8, the bridging principles set out in subsections (3) to (8) must be applied if there is an indication to that effect.
Dilution
(3) If a tested mixture that is classified in a category or subcategory of a health hazard class set out in Subparts 1 to 10 of Part 8 is diluted with a diluent, the following applies provided that the diluent is a mixture or substance that, with respect to that health hazard class, has an equivalent or less severe hazard classification than the least hazardous ingredient of the tested mixture and, based on established scientific principles, does not affect the classification of the tested mixture:
- (a) in the case of a tested mixture that is classified in a category or subcategory of a health hazard class set out in Subparts 1 to 3 of Part 8, either the method referred to in section 8.1.5, 8.2.11 or 8.3.11, as the case may be, must be used to establish whether the diluted mixture must be classified in a category or subcategory of a hazard class, or the diluted mixture must be classified in the same category or subcategory of the health hazard class as the tested mixture; or
- (b) in all other cases, the diluted mixture must be classified in the same category or subcategory of the health hazard class as the tested mixture.
Production batches
(4) The classification is the same for a mixture in all production batches of that mixture that are manufactured, produced or processed by the same supplier, unless there is a significant variation between the batches that affects the classification of the mixture.
Increase in concentration of hazardous ingredient
(5) If the concentration of a hazardous ingredient of a tested mixture is increased, the following applies:
- (a) in the case of the health hazard classes set out in Subparts 1, 4 and 8 to 10 of Part 8, if the tested mixture is classified in the Category 1 category of the health hazard class, the new mixture resulting from the increased concentration must be classified in the same category of the same health hazard class, without additional evaluation with regard to that hazard class;
- (b) in the case of the health hazard class set out in Subpart 2 of Part 8,
- (i) if the tested mixture is classified in the Category 1A subcategory of the health hazard class, the new mixture resulting from the increased concentration must be classified in the same subcategory of the same health hazard class, without additional evaluation with regard to that hazard class, or
- (ii) if the tested mixture does not contain any hazardous ingredient classified in the Category 1 category and is classified in the Category 2 category of the health hazard class, the new mixture resulting from the increased concentration must be classified in the same category of the same health hazard class, without additional evaluation with regard to that hazard class; and
- (c) in the case of the health hazard class set out in Subpart 3 of Part 8,
- (i) if the tested mixture is classified in the Category 1 category of the health hazard class, the new mixture resulting from the increased concentration must be classified in the same category of the same health hazard class, without additional evaluation with regard to that hazard class, or
- (ii) if the tested mixture does not contain any hazardous ingredient classified in the Category 1 category and is classified in the Category 2A subcategory of the health hazard class, the new mixture resulting from the increased concentration must be classified in the same subcategory of the same health hazard class, without additional evaluation with regard to that hazard class.
Interpolation
(6) In the case of the health hazard classes set out in Subparts 1 to 4 and 8 to 10 of Part 8, when three mixtures (A, B and C) contain identical ingredients — some or all of which are hazardous — if mixtures A and B have been tested and are classified in the same category or subcategory of the same health hazard class and if mixture C has not been tested and has the same hazardous ingredients as mixtures A and B with concentrations intermediate to the concentrations of those hazardous ingredients in mixtures A and B, then mixture C must be classified in the same category or subcategory of the same health hazard class as mixtures A and B.
Substantially similar mixtures
(7) If one of the mixtures (ingredient A + ingredient B) or (ingredient C + ingredient B) is a tested mixture that is classified in a category or subcategory of a health hazard class, the other mixture must be classified in the same category or subcategory of the same health hazard class if the following conditions are met:
- (a) the concentration of ingredient B is the same in both mixtures;
- (b) the concentration of ingredient A is the same as that of ingredient C; and
- (c) ingredients A and C are classified in the same category or subcategory of the same health hazard class and, based on established scientific principles, do not affect the classification of ingredient B.
Aerosols — health hazard classes
(8) In the case of the health hazard classes set out in Subparts 1 to 4, 8 and 9 of Part 8, a mixture to which a propellant has been added and that is contained in an aerosol dispenser must be classified in the same category or subcategory of the same health hazard class as the mixture to which no propellant was added if, in accordance with established scientific principles, the added propellant does not affect the classification of the mixture on spraying.
Other Principles
Synergistic effects
2.4. (1) In order to establish whether a mixture is classified in a category or subcategory of a health hazard class, if the evaluation of the mixture is carried out in accordance with a provision that requires the use of data available on the ingredients in the mixture, then all data available on the potential occurrence of synergistic effects among the ingredients of the mixture must be used in the evaluation carried out in accordance with section 2.2.
Antagonistic effects
(2) If antagonistic effects among the ingredients of the mixture are considered in order to establish the classification of the mixture in a category or subcategory of a health hazard class in the course of the evaluation carried out in accordance with section 2.2, the data in respect of the antagonistic effects must be conclusive, in accordance with established scientific principles.
Concentration limits — lower concentration
2.5. (1) In the case of Subparts 1 to 10 and 12 of Part 8, if an ingredient is present in a mixture at a lower concentration than the concentration limit for a particular category or subcategory of a health hazard class, but still presents the hazard identified by the category or subcategory of that hazard class at that concentration, the mixture must be classified in that category or subcategory.
Concentration limits — equivalent or higher concentration
(2) In the case of Subparts 1 to 10 and 12 of Part 8, subject to subsection 2.4(1), if an ingredient is present in a mixture at an equivalent or higher concentration than the concentration limit for a particular category or subcategory of a health hazard class, but further to evidence based on established scientific principles it does not present the hazard identified by the category or subcategory of that hazard class at that concentration, the mixture need not be classified in that category or subcategory in relation to that specific ingredient.
Maximum concentration
2.6. If a mixture with a specific product identifier contains a hazardous ingredient that is not always present at the same concentration, the maximum concentration must be used for the purposes of establishing whether the mixture is classified in a category or subcategory of a health hazard class.
PRODUCT
Classification — product
2.7. Subject to section 2.9, to establish whether a product is classified in a category or subcategory of a physical hazard class, it must be evaluated in accordance with section 2.1 or 2.2.
SPECIFIC RULES
Biological availability
2.8. If it can be shown by conclusive experimental data from scientifically validated methods that the mixture, material or substance is not biologically available, it need not be classified in any health hazard class.
Solids
2.9. In the case of the physical hazard classes set out in Subparts 7, 10 to 12 and 14 of Part 7, the data used for the purposes of evaluation of a solid must relate to the solid in the physical form in which it is sold or imported. If the solid is in a physical form that is different from that used to generate the data and the solid in that physical form is liable to display different behaviour, the solid must also be evaluated in that other physical form.
PART 3
LABELLING
Information elements
3. (1) Subject to section 3.6 and for the purposes of paragraphs 13(1)(b) and 14(b) of the Act, the label of a hazardous product or the container in which the hazardous product is packaged must provide, in respect of the hazardous product, the following information elements:
- (a) the product identifier;
- (b) the initial supplier identifier;
- (c) subject to subsections (2) to (6), for each category or subcategory in which the hazardous product is classified, with the exception of the categories referred to in paragraph (d), the information elements that are specified for that category or subcategory in section 3 of Annex 3 of the GHS;
- (d) subject to subsections (2) to (5), for each category set out in Subparts 17 to 20 of Part 7 and in Subparts 11 and 12 of Part 8 in which the hazardous product is classified,
- (i) the information elements that are specified for that category in Schedule 5, and
- (ii) any precautionary statements that are applicable to the hazardous product in terms of
- (A) general precautionary statements,
- (B) prevention precautionary statements,
- (C) response precautionary statements,
- (D) storage precautionary statements, and
- (E) disposal precautionary statements;
- (e) in the case of a hazardous product classified in a category of Subpart 1 of Part 8 and to which paragraph 8.1.6(b) applies, the supplemental label element “[Insert the total concentration in percentage of ingredients with unknown acute toxicity] % of the mixture consists of an ingredient or ingredients of unknown acute toxicity/[Insérez la concentration totale en pourcentage d'ingrédients ayant une toxicité aiguë inconnue] % du mélange consiste en ingrédients de toxicité aiguë inconnue”; and
- (f) in the case of a hazardous product that is classified as an acute toxicant and that, upon contact with water, releases a gaseous substance that has an LC50 that falls into one of the ranges indicated in table 3 to subsection 8.1.1(3), the supplemental label elements that consist of the following hazard statements:
- (i) in the case of Categories 1 and 2, “In contact with water, releases gases which are fatal if inhaled/Au contact de l'eau, libère des gaz mortels en cas d'inhalation”,
- (ii) in the case of Category 3, “In contact with water, releases gases which are toxic if inhaled/Au contact de l'eau, libère des gaz toxiques en cas d'inhalation”, or
- (iii) in the case of Category 4, “In contact with water, releases gases which are harmful if inhaled/Au contact de l'eau, libère des gaz nocifs en cas d'inhalation”.
Codes or instructions
(2) The information elements required by paragraph (1)(c) need not include alphanumeric codes and the information elements required by paragraphs (1)(c) and (d) must not include instructions that are for the exclusive use of the competent authority, as defined in the GHS, or the supplier.
Substitution by pictogram
(3) The pictogram associated with a symbol in Schedule 3 must be substituted for the symbol that is specified for a category or subcategory in section 3 of Annex 3 of the GHS or for a category in Schedule 5.
Information for certain hazard statements
(4) Information required by the instructions in italics and in parentheses in the hazard statements specified in section 3 of Annex 3 of the GHS in respect of the hazard classes “Germ Cell Mutagenicity”, “Carcinogenicity”, “Reproductive Toxicity”, “Specific Target Organ Toxicity — Single Exposure” and “Specific Target Organ Toxicity — Repeated Exposure” must not be specified on the label unless all applicable organs, effects and routes of exposure are stated.
Hazard statement — Specific Target Organ Toxicity — Single Exposure
(5) In the case of a hazardous product that is classified in the category “Specific Target Organ Toxicity — Single Exposure — Category 3” of the hazard class “Specific Target Organ Toxicity — Single Exposure”, the hazard statement specified for that category in section 3 of Annex 3 of the GHS that relates to the effects for which the product was classified must be used. If the hazardous product causes narcotic effects and respiratory tract irritation, as those terms are defined in Subpart 8 of Part 8, then both hazard statements must be used.
Information elements for certain categories or subcategories
(6) The information elements specified in section 3 of Annex 3 of the GHS to be used for hazardous products classified in the categories or subcategories specified below are as follows:
- (a) if the hazardous product is classified in the category “Skin Corrosion — Category 1”, the information elements specified for the subcategory “Skin Corrosion — Category 1A”;
- (b) if the hazardous product is classified in the category “Eye Irritation — Category 2”, the information elements specified for the subcategory “Eye Irritation — Category 2A”; and
- (c) if the hazardous product is classified in the subcategory “Carcinogenicity — Category 1A” or in the subcategory “Carcinogenicity — Category 1B”, the information elements specified for the category “Carcinogenicity — Category 1”.
Pictograms
3.1. Any pictogram required to be provided on a label must, except with respect to size, be an exact reproduction of that pictogram as set out in column 3 of Schedule 3 and must,
- (a) except for the pictogram for “Biohazardous Infectious Materials”, have a black symbol on a white background with a red border in the shape of a square set on one of its points; and
- (b) in the case of the pictogram for “Biohazardous Infectious Materials”, have a black symbol on a white background with a black border in the shape of a circle.
Combined precautionary statements
3.2. (1) The precautionary statements that are required to be provided on a label may be combined if the combination contains the same information as would have been conveyed by each of the individual precautionary statements.
Non-applicable precautionary statements
(2) If a precautionary statement does not apply in a particular case with regard to the normal conditions of use, handling and storage of the hazardous product, it may be omitted.
Combined hazard statements
(3) The hazard statements that are required to be provided on a label may be combined if the combination contains the same information as would have been conveyed by each of the individual hazard statements.
Information elements of label
3.3. The pictogram, signal word and hazard statement must be grouped together on the label.
Legibility
3.4. The information elements of the label of the hazardous product or container in which it is packaged must be clearly and prominently displayed on a surface that is visible under normal conditions of use, easily legible without the aid of any device other than corrective lenses and contrasted with any other information on the hazardous product or the container.
Durability
3.5. The information elements of the label of the hazardous product or container in which it is packaged must, under normal conditions of transport and use, remain affixed to, printed or written on or attached to the hazardous product or the container and remain legible.
Specific rule — signal word
3.6. (1) If there is a requirement to provide the signal word “Danger”, any requirement to provide the signal word “Warning” does not apply.
Specific rule — hazard statement
(2) If there is a requirement to provide the hazard statement “Causes severe skin burns and eye damage”, any requirement to provide the hazard statement “Causes serious eye damage” does not apply.
Specific rule — symbol
(3) In the case of the symbols specified below, the following apply:
- (a) if there is a requirement to provide the “skull and crossbones” symbol, any requirement to provide the “exclamation mark” symbol to indicate acute toxicity does not apply;
- (b) if there is a requirement to provide the “corrosion” symbol, any requirement to provide the “exclamation mark” symbol to indicate skin or eye irritation does not apply; and
- (c) if there is a requirement to provide the “health hazard” symbol to indicate respiratory sensitization, any requirement to provide the “exclamation mark” symbol to indicate skin sensitization or skin or eye irritation does not apply.
PART 4
SAFETY DATA SHEET
Information elements
4. (1) For the purposes of paragraphs 13(1)(a) and 14(a) of the Act, the safety data sheet of a hazardous product must provide, in respect of the hazardous product, the following information elements:
- (a) the headings set out in column 1 of Schedule 1, in the order they are presented, including the corresponding item number, which is to be placed immediately before the heading;
- (b) subject to section 4.5, the content of the specific information elements set out in paragraphs 3(1)(a) and (2)(a) and (d) of Schedule 1 for the heading for item 3 and, for each heading of that Schedule, if the information is available and applicable, the content of the other specific information elements of that Schedule, including the unit of measure, if applicable, taking into account the following:
- (i) subject to subsection (2), if any of the information — except that required by paragraphs 3(1)(a) and (2)(a) and (d) of that Schedule — is not available or not applicable, an indication to that effect must be clearly stated in lieu of the required specific information element, and
- (ii) in the case of a mixture, the information provided under the heading for item 11 of Schedule 1 must be information that is available on the mixture as a whole, and if information is not available on the mixture as a whole, it must be information that is available on the hazardous ingredients in the mixture, together with a clear indication of the chemical name of the hazardous ingredient to which the information pertains; and
- (c) under any applicable heading, all additional hazard information that is available with respect to
- (i) the hazardous product, and
- (ii) a product, mixture, material or substance that has similar properties, including any evidence based on established scientific principles, if that information is applicable to the normal conditions of use of the hazardous product and is not redundant, indicated alongside an identification of the product, mixture, material or substance that has similar properties.
Item 11 of Schedule 1
(2) With respect to the heading for item 11 of Schedule 1, an indication to the effect that the specific information element required by that item is not applicable must not appear.
Items 12 to 15 of Schedule 1
(3) Despite subsection (1), under each heading set out for items 12 to 15 of Schedule 1, the content of the specific information elements in that Schedule may be omitted.
Biohazardous Infectious Materials — additional information elements
(4) The following information elements must be provided, immediately following the information elements required by subsection (1), on the safety data sheet of a hazardous product that is classified in a category of the hazard class “Biohazardous Infectious Materials”:
- (a) the headings set out in Schedule 2, in the order they are presented;
- (b) under each heading, the name of each specific information element set out in column 2 in respect of that heading in the order they are presented;
- (c) under the name of each specific information element, the content of the information element, if the information is available and applicable, including the unit of measure, if applicable, taking into account the following:
- (i) if any of the information is not available or not applicable, an indication to that effect must be clearly stated in lieu of the required information, and
- (ii) any information provided under one heading of the safety data sheet need not be repeated under any other heading.
More than one biohazardous infectious material
(5) In the case where a mixture contains more than one ingredient that is classified as a biohazardous infectious material, the information required by subsection (4) must be provided in distinct parts on the safety data sheet, sequentially, for each biohazardous infectious material.
Information elements — new substance
4.1. In the case of a hazardous product — for which instructions for use, provided at the time of sale or import, require its combination with one or more products, mixtures, materials or substances and for which that combination results in the creation of one or more new substances, mixtures or materials — the safety data sheet of the hazardous product must also provide, in respect of any resulting new substance, mixture or material, under each applicable heading, the content of the specific information elements set out in column 2 of Schedule 1 in respect of that heading that is available in relation to the hazardous properties of that new substance, mixture or material and the ways to protect against its hazards, and clearly indicate that the information pertains to the new substance, mixture or material.
Identical identifiers
4.2. The product identifier and the initial supplier identifier that are provided on the safety data sheet of a hazardous product must be identical to those provided on the label.
Concentration units
4.3. If the concentration of a material or substance in a hazardous product is expressed as a percentage on the safety data sheet, the units used to calculate the percentage must be provided.
Most hazardous concentration
4.4. If ingredients in a mixture that is a hazardous product are present in a range of concentrations, the information provided on the safety data sheet must be based on data available that correspond to the most hazardous concentration of each ingredient in the mixture, whether those data pertain to an ingredient or the mixture as a whole.
Concentration ranges
4.5. If the concentration of a material or substance in a hazardous product is required to be provided on a safety data sheet and the material or substance is not always present at the same concentration, the safety data sheet must provide, in lieu of the concentration of the material or substance, the actual concentration range of the material or substance in the hazardous product.
PART 5
EXCEPTIONS
Definition of “laboratory sample”
5. (1) In this section, “laboratory sample” means a sample of a hazardous product that is packaged in a container that contains less than 10 kg of the hazardous product and that is intended solely to be tested in a laboratory, but does not include a sample that is to be used
- (a) by the laboratory for testing other products, mixtures, materials or substances; or
- (b) for educational or demonstration purposes.
Sale or importation — biohazardous infectious materials — safety data sheet
(2) Subject to subsection (3), the sale or importation of a laboratory sample that is classified only in the category “Biohazardous Infectious Materials — Category 1” is exempt from the application of paragraphs 13(1)(a) and (a.1) and 14(a) of the Act.
Transfer of possession — biohazardous infectious materials — safety data sheet and label
(3) The transfer of possession of a laboratory sample for a specific purpose, without transferring ownership, if that laboratory sample is classified only in the category “Biohazardous Infectious Materials — Category 1”, is exempt from the application of section 13 of the Act.
Transfer of possession — safety data sheet
(4) The transfer of possession of a laboratory sample for a specific purpose, without transferring ownership, if that laboratory sample is one of the following types, is exempt from the application of paragraphs 13(1)(a) and (a.1) of the Act:
- (a) a laboratory sample for which the chemical name and concentration of the hazardous product or its ingredients are not known; or
- (b) a laboratory sample for which the supplier has not offered or exposed the hazardous product for transfer of ownership.
Sale or importation — biohazardous infectious materials — label
(5) Subject to subsection (3), the sale or importation of a laboratory sample that is classified only in the category “Biohazardous Infectious Materials — Category 1” is exempt from the application of paragraph 3(1)(d) if the label provides the chemical name or generic chemical name of any material that is in the hazardous product and that is classified as a Biohazardous Infectious Material, if known by the supplier, and the statement “Hazardous Laboratory Sample. For hazard information or in an emergency, call / Échantillon pour laboratoire de produit dangereux. Pour obtenir des renseignements sur les dangers ou en cas d'urgence, composez”, followed by an emergency telephone number for the purpose of obtaining the information elements that must be provided on the safety data sheet of the hazardous product.
Transfer of possession — label
(6) The transfer of possession of a laboratory sample for a specific purpose, without transferring ownership, is exempt from the application of paragraphs 3(1)(c) and (d) if the label provides the chemical name or generic chemical name of any material or substance that is in the hazardous product and that is referred to in subsection 3(2) of Schedule 1, if known by the supplier, and the statement “Hazardous Laboratory Sample. For hazard information or in an emergency, call / Échantillon pour laboratoire de produit dangereux. Pour obtenir des renseignements sur les dangers ou en cas d'urgence, composez”, followed by an emergency telephone number for the purpose of obtaining the information elements that must be provided on the safety data sheet of the hazardous product, and if that laboratory sample is one of the following types:
- (a) a laboratory sample for which the chemical name and concentration of the hazardous product or its ingredients are not known; or
- (b) a laboratory sample in respect of which the supplier has not offered or exposed the hazardous product for transfer of ownership.
Mixture of radioactive nuclides and non-radioactive carriers — section 13 or 14 of Act
5.1. (1) The sale or importation of a hazardous product that is a mixture of one or more radioactive nuclides and one or more non-radioactive carriers is exempt from the application of section 13 or 14 of the Act if the carrier
- (a) is present in an amount that is
- (i) in the case of a liquid or gaseous carrier, less than or equal to 1.0 ml , or
- (ii) in the case of a solid carrier, less than or equal to 1.0 g ; and
- (b) is not
- (i) classified in any category or subcategory of the “Carcinogenicity”, “Germ Cell Mutagenicity”, “Reproductive Toxicity” or “Biohazardous Infectious Materials” hazard class,
- (ii) classified in the category “Acute Toxicity (Oral) — Category 1” or “Acute Toxicity (Dermal) — Category 1” of the “Acute Toxicity” hazard class, or
- (iii) classified in the category “Acute Toxicity (Inhalation) — Category 1” or “Acute Toxicity (Inhalation) — Category 2” of the “Acute Toxicity” hazard class.
Mixture of radioactive nuclides and non-radioactive carriers — paragraph 13(1)(b) or 14(b) of Act
(2) The sale or importation of a hazardous product that is a mixture of one or more radioactive nuclides and one or more non-radioactive carriers is exempt from the application of paragraph 13(1)(b) or 14(b) of the Act in respect of the requirement to have a label on the inner container of the hazardous product if the hazardous product is packaged in more than one container and the outer container has a label that provides the information elements required by Part 3.
Mixture of radioactive nuclides and non-radioactive carriers
(3) The sale or importation of a hazardous product that is a mixture of one or more radioactive nuclides and one or more non-radioactive carriers is exempt from the application of
- (a) paragraph 3(1)(b); and
- (b) paragraph 3(1)(c) and subparagraph 3(1)(d)(ii) in respect of the requirement to provide any precautionary statement on the label of the hazardous product or the container in which it is packaged.
Outer container
5.2. The sale or importation of a hazardous product is exempt from the application of paragraph 13(1)(b) or 14(b) of the Act in respect of the requirement to have a label on the outer container of the hazardous product if
- (a) the label on the inner container is visible and legible through the outer container under normal conditions of storage and handling; or
- (b) the outer container has a label that meets the requirements set out in the Transportation of Dangerous Goods Regulations.
Label — outer container — at least two hazardous products
5.3. In the case of an outer container in which at least two different hazardous products are packaged, subsection 3(1) does not apply if the label provides the following information elements:
- (a) the product identifier for each hazardous product contained in the outer container;
- (b) the initial supplier identifier;
- (c) subject to subsection 3.6(3), the pictogram set out in column 3 of Schedule 3 designated for each category or subcategory in which each hazardous product contained in the outer container is classified;
- (d) the precautionary statement applicable to the storage of each of the hazardous products contained in the outer container; and
- (e) the statement “See individual product labels for signal words, hazard statements and precautionary statements/Voir les étiquettes sur chacun des produits pour les mentions d'avertissement, les mentions de danger et les conseils de prudence”.
Small-capacity containers — 100 ml or less
5.4. (1) The sale or importation of a hazardous product in a container that has a capacity of less than or equal to 100 ml, including any subsequent container of the same capacity in which that first container is packaged, is exempt from the application of paragraph 3(1)(c) and subparagraph 3(1)(d)(i) or (ii) in respect of the requirement to provide any precautionary statement or hazard statement on the label of the hazardous product or the container.
Small-capacity containers — 3 ml or less
(2) The sale or importation of a hazardous product in a container that has a capacity of less than or equal to 3 ml is exempt from the application of section 3.5 in respect of normal conditions of use if the label interferes with the normal use of the hazardous product.
Definition of “bulk shipment”
5.5. (1) In this section, “bulk shipment” means a shipment of a hazardous product that is contained in any of the following, without intermediate containment or intermediate packaging:
- (a) a vessel that has a water capacity equal to or greater than 450 l;
- (b) a freight container, road vehicle, railway vehicle or portable tank;
- (c) the hold of a ship; or
- (d) a pipeline.
Bulk shipments and unpackaged hazardous products
(2) The sale or importation of a bulk shipment or a hazardous product without packaging of any sort is exempt from the application of paragraph 13(1)(b) or 14(b) of the Act.
Definition of “complex mixture”
5.6. (1) In this section, “complex mixture” means a mixture that has a commonly known generic name and that is
- (a) naturally occurring;
- (b) a fraction of a naturally occurring mixture that results from a separation process; or
- (c) a modification of a naturally occurring mixture or a modification of a fraction of a naturally occurring mixture that results from a chemical modification process.
Complex mixture
(2) The sale or importation of a hazardous product that is a complex mixture is exempt from the application of paragraph 4(1)(b) in respect of the requirements set out in paragraphs 3(2)(a) and (d) of Schedule 1, and in paragraphs 3(2)(b) and (c) of that Schedule if that information is available and applicable, if the commonly known generic name of the complex mixture is provided for item 3 of the safety data sheet.
Complex mixture — ingredient
(3) Subject to subsection (4), the sale or importation of a hazardous product that contains an ingredient that is a complex mixture is exempt from the application of paragraph 4(1)(b) in respect of the requirements set out in paragraphs 3(2)(a) and (d) of Schedule 1, and in paragraphs 3(2)(b) and (c) of that Schedule, if that information is available and applicable, in relation to the ingredients of the complex mixture if
- (a) the complex mixture, individually, is classified in a category or subcategory of a health hazard class and the commonly known generic name of the complex mixture and its concentration in the hazardous product are provided for item 3 of the safety data sheet;
- (b) the complex mixture is present in the hazardous product at a concentration of less than 0.1% and is classified in any of the following categories or subcategories:
- (i) Carcinogenicity — Category 1A, 1B or 2,
- (ii) Reproductive Toxicity — Category 1A, 1B or 2,
- (iii) Reproductive Toxicity — Effects on or via lactation,
- (iv) Respiratory Sensitization — Category 1A, or
- (v) Germ Cell Mutagenicity — Category 1A or 1B;
- (c) the complex mixture is not referred to in paragraph (b) and is present in the hazardous product at a concentration of less than 0.2% and is a gas classified in the subcategory “Respiratory Sensitization — Category 1B”; or
- (d) the complex mixture is not referred to in paragraph (b) or (c) and is present in the hazardous product at a concentration of less than 1.0%.
Concentration results in classification
(4) If the complex mixture is present at a concentration that results in the product being classified in a category or subcategory of any health hazard class further to subsection 2.5(1), the commonly known generic name and concentration of the complex mixture must be provided on the safety data sheet of the hazardous product.
Definitions
5.7. (1) The following definitions apply in this section.
“first supplier”
« premier fournisseur »
“first supplier” means a supplier who is exempted from disclosing the information specified in subsection 11(1) of the Hazardous Materials Information Review Act, by virtue of that Act.
“subsequent supplier”
« fournisseur subséquent »
“subsequent supplier” means a supplier who sells or imports a hazardous product that is the subject of an exemption granted to the first supplier from the requirement to disclose the information specified in subsection 11(1) of the Hazardous Materials Information Review Act.
Confidential information
(2) If any information is the subject of an exemption under the Hazardous Materials Information Review Act, the information must be replaced by the information required under subsection (3) or (4).
Subsection 11(1) — Hazardous Materials Information Review Act
(3) A supplier who, under subsection 11(1) of the Hazardous Materials Information Review Act, files a claim for exemption from a requirement to disclose information in respect of a hazardous product on a safety data sheet or on a label must, in respect of the sale or importation of the hazardous product, provide on the safety data sheet and, if applicable, on the label of the hazardous product or container in which the hazardous product is packaged a statement that a claim was filed, the date that the claim was filed and the registry number assigned to the claim under the Hazardous Materials Information Review Act until
- (a) in the case that an order was issued by a screening officer under subsection 16(1) or 17(1) of the Hazardous Materials Information Review Act, the end of the period that begins on the final disposition of the proceedings in relation to the claim for exemption and does not exceed the period specified in the order, as the word “proceedings” is defined in subsection 19(3) of the Hazardous Materials Information Review Act; or
- (b) in any other case, the end of the period not exceeding 30 days after the final disposition of the proceedings in relation to the claim for exemption, as the word “proceedings” is defined in subsection 19(3) of the Hazardous Materials Information Review Act.
Information to be disclosed
(4) A supplier who receives notice of a decision made under the Hazardous Materials Information Review Act that their claim or a portion of their claim for exemption from a requirement to disclose information in respect of a hazardous product on a safety data sheet or a label is valid must, during the period beginning no later than the end of the applicable period specified in subsection (3) and on compliance with any order issued under subsection 16(1) or 17(1) of the Hazardous Materials Information Review Act, if applicable, and ending on the last day of the exemption period, in respect of the sale or importation of the hazardous product, provide on the safety data sheet and, if applicable, on the label of the hazardous product or container in which the hazardous product is packaged the following information:
- (a) a statement that an exemption has been granted;
- (b) the date of the decision granting the exemption; and
- (c) the registry number assigned to the claim under the Hazardous Materials Information Review Act.
Non- application — paragraphs 3(1)(a) to (d) or (2)(a) to (c) of Schedule 1
(5) The sale or importation of a hazardous product is exempt from the application of paragraph 4(1)(b) in respect of the requirements set out in paragraph 3(1)(a) or (2)(a) of Schedule 1, and in paragraphs 3(1)(b) to (d) or 2(b) and (c) of that Schedule if the information is available and applicable, if it is the subject of a claim for exemption under paragraph 11(1)(a) of the Hazardous Materials Information Review Act and if the generic chemical name of the material, substance or ingredient is provided for item 3 of the safety data sheet.
Non- application — paragraph 3(2)(d) of Schedule 1
(6) Paragraph 3(2)(d) of Schedule 1 does not apply in respect of a hazardous product that is the subject of a claim for exemption under subparagraph 11(1)(b)(iii) of the Hazardous Materials Information Review Act.
Sale or importation — paragraphs 3(1)(a) to (d) or (2)(a) to (c) of Schedule 1
(7) The sale or importation of a hazardous product by a subsequent supplier is exempt from the application of paragraph 4(1)(b) in respect of the requirements set out in paragraph 3(1)(a) or (2)(a) of Schedule 1, and in paragraphs 3(1)(b) to (d) or 2(b) and (c) of that Schedule if the information is available and applicable, if the first supplier is exempt from these requirements and if the information is unknown to the subsequent supplier or the information is known to the subsequent supplier and that supplier has obtained the information in confidence, express or implied, and is obligated, expressly or implicitly, by contract or a relationship based on trust and confidence, or otherwise by law or equity, to maintain the confidentiality of the information and
- (a) the safety data sheet for the hazardous product provided by the subsequent supplier on the sale provides, in lieu of the information referred to in paragraph 3(1)(a) or (2)(a) of Schedule 1, and in paragraphs 3(1)(b) to (d) or 2(b) and (c) of that Schedule if the information is available and applicable,
- (i) the information referred to in subsection (3) or (4) in respect of,
- (A) if the subsequent supplier is exempted from the requirement to provide information that could be used to identify the first supplier, that exemption, or
- (B) in any other case, the exemption of the first supplier, with the words “other supplier” in parentheses after that information, and
- (ii) the generic chemical name of the material, substance or ingredient as provided by that supplier; and
- (i) the information referred to in subsection (3) or (4) in respect of,
- (b) the subsequent supplier provides, with the safety data sheet for the hazardous product, the safety data sheet provided by the first supplier.
Sale or importation — paragraph 3(2)(d) of Schedule 1
(8) The sale or importation of a hazardous product by a subsequent supplier is exempt from the application of paragraph 4(1)(b) in respect of the requirement set out in paragraph 3(2)(d) of Schedule 1 if the first supplier is exempt from that requirement and if the information is unknown to the subsequent supplier or the information is known to the subsequent supplier and that supplier has obtained the information in confidence, express or implied, and is obligated, expressly or implicitly, by contract or a relationship based on trust and confidence, or otherwise by law or equity, to maintain the confidentiality of the information and
- (a) the safety data sheet for the hazardous product provided by the subsequent supplier on the sale provides, in lieu of the information referred to in paragraph 3(2)(d) of Schedule 1,
- (i) the information referred to in subsection (3) or (4) in respect of,
- (A) if the subsequent supplier is exempted from the requirement to provide information that could be used to identify the first supplier, that exemption, or
- (B) in any other case, the exemption of the first supplier, with the words “other supplier” in parentheses after that information, and
- (ii) subject to section 4.5, the concentration of the first supplier's hazardous product that is in the subsequent supplier's hazardous product; and
- (i) the information referred to in subsection (3) or (4) in respect of,
- (b) the subsequent supplier provides, with the safety data sheet for the hazardous product, the safety data sheet provided by the first supplier.
Label — confidential product identifier — paragraphs 3(1)(a) and 4(1)(b)
(9) Paragraph 3(1)(a) and the requirement in paragraph 4(1)(b) in relation to paragraph 1(a) of Schedule 1 if the information is available and applicable do not apply in respect of the sale of a hazardous product to an employer who is exempt under the Hazardous Materials Information Review Act or under the laws of a province from the requirement to disclose the product identifier of a hazardous product if the label provides a code name or code number specified by the supplier and
- (a) if available, the information referred to in subsection (3) or (4) in respect of the employer's claim for exemption under the Hazardous Materials Information Review Act; or
- (b) if the information referred to in paragraph (a) is not available, the information required to be provided under the laws of the province.
Label — confidential supplier identifier — paragraphs 3(1)(b) and 4(1)(b)
(10) Paragraph 3(1)(b) and the requirement in 4(1)(b) in relation to paragraph 1(d) of Schedule 1 if the information is available and applicable do not apply in respect of the sale of a hazardous product to an employer who is exempt under the Hazardous Materials Information Review Act or under the laws of a province from the requirement to disclose any information that could be used to identify the supplier of the hazardous product if that information is replaced by
- (a) if available, the information referred to in subsection (3) or (4) in respect of the employer's claim for exemption under the Hazardous Materials Information Review Act; or
- (b) if the information referred to in paragraph (a) is not available, the information required to be provided under the laws of the province.
Safety data sheet — sale to employer
(11) The sale of a hazardous product to an employer is exempt from the requirement to disclose information on the safety data sheet that could be the subject of a claim for exemption under subsection 11(2) of the Hazardous Materials Information Review Act if
- (a) the employer is exempt, under that Act or the laws of a province, from disclosing that information in respect of the hazardous product; and
- (b) the safety data sheet of the hazardous product provided in respect of that sale provides in lieu of that information
- (i) if available, the information referred to in subsection (3) or (4) in respect of the employer's claim for exemption under that Act, or
- (ii) if the information referred to in subparagraph (i) is not available, an emergency telephone number of the employer that will enable a health professional to obtain any information referred to in subsection 4(1) that is in the possession of the employer for the purpose of making a medical diagnosis of, or rendering medical treatment to, a person in an emergency.
Subsequent sale by supplier — safety data sheet
5.8. (1) The sale of a hazardous product by a supplier to whom the hazardous product was sold is exempt from the application of paragraph 4(1)(b) in respect of the requirement set out in paragraph 1(d) of Schedule 1 to provide the initial supplier identifier on the safety data sheet if their name, address and telephone number are provided on the safety data sheet.
Subsequent sale by supplier — label
(2) The sale of a hazardous product by a supplier to whom the hazardous product was sold is exempt from the application of paragraph 3(1)(b) in respect of the requirement to provide the initial supplier identifier on the label if their name, address and telephone number are provided on the label.
Following supplier
(3) If the initial supplier identifier referred to in subsection (1) or (2) has been replaced by the name, address and telephone number of a supplier to whom the hazardous product has been sold, any following supplier of the hazardous product may replace that information with their own name, address and telephone number.
Importation for use in own work place — safety data sheet
5.9. (1) If an importer imports a hazardous product from a foreign supplier for use in their own work place in Canada and obtains a safety data sheet from the foreign supplier, the importer is exempt from the requirement to provide, on the safety data sheet, the specific information element set out in paragraph 1(d) of Schedule 1 if the name, address and telephone number of the foreign supplier is retained on the safety data sheet.
Importation for use in own work place — label
(2) If an importer imports a hazardous product from a foreign supplier for use in their own work place in Canada, the importer is exempt from the application of paragraph 3(1)(b) in respect of the requirement to provide the initial supplier identifier on the label if the name, address and telephone number of the foreign supplier is retained on the label.
Repetition of symbols on label
5.10. The sale or importation of a hazardous product is exempt from the application of paragraphs 3(1)(c) and (d) in respect of the requirement to provide a pictogram on the label of the hazardous product or its container if the symbol of the pictogram appears on another label in accordance with the Transportation of Dangerous Goods Regulations on that same hazardous product or its container, and if the other label also meets the requirements of section 3.5.
Safety data sheet for hazardous products — same product identifier
5.11. The sale or importation of a hazardous product is exempt from the application of paragraph 13(1)(a.1) or 14(a) of the Act in respect of the requirement to provide, or cause to be provided, on the sale a safety data sheet or to obtain or prepare a safety data sheet on or before the importation, if
- (a) the hazardous product is part of a shipment of hazardous products that have the same product identifier and a safety data sheet is obtained, prepared or provided for one of them; or
- (b) the supplier has provided to the person or government who acquires possession or ownership, or the supplier who imports the hazardous product has in their possession, a safety data sheet for a hazardous product that has the same product identifier and the safety data sheet provides, subject to section 5.12, information that is current at the time of the sale or importation.
Definition of “significant new data”
5.12. (1) In this section, “significant new data” means new data regarding the hazard presented by a hazardous product that change its classification in a category or subcategory of a hazard class, or result in its classification in another hazard class, or change the ways to protect against the hazard presented by the hazardous product.
Significant new data available within 90 days — sale
(2) The sale of a hazardous product for which significant new data became available within 90 days prior to the sale is exempt from the application of subsection 4(1) in respect of the requirement to provide, on the safety data sheet, information that is available at the time of sale if, at the time of the sale, the supplier ensures that the person or government that acquires possession or ownership is provided with
- (a) a safety data sheet that includes all information available at the time of sale, with the exception of the significant new data; and
- (b) the significant new data and the date on which they became available, in writing.
Significant new data available within 90 days — importation
(3) The importation of a hazardous product for which significant new data became available within 90 days prior to the importation is exempt from the application of subsection 4(1) in respect of the requirement to provide, on the safety data sheet, information that is available at the time of the importation if, at the time of importation, the supplier
- (a) obtains a safety data sheet that includes all of the information available at the time of importation, with the exception of the significant new data; and
- (b) obtains or prepares a document that provides the significant new data and the date on which they became available and appends that document to the safety data sheet referred to in paragraph (a).
Significant new data available within 180 days — sale
(4) The sale of a hazardous product for which significant new data became available within 180 days prior to the sale is exempt from the application of subsection 3(1) in respect of the requirement to provide, on the label, information elements for each category or subcategory of the hazard class in which the hazardous product is classified at the time of the sale if, at the time of the sale,
- (a) the hazardous product or container in which the hazardous product is packaged has a label that provides all the information elements for each category or subcategory of the hazard class in which the hazardous product is classified at the time of sale, with the exception of the significant new data; and
- (b) the person or government that acquires possession or ownership is provided with the significant new data and the date on which they became available, in writing.
Significant new data available within 180 days — importation
(5) The importation of a hazardous product for which significant new data became available within 180 days prior to the importation is exempt from the application of subsection 3(1) in respect of the requirement to provide, on the label, information elements for each category or subcategory of the hazard class in which the hazardous product is classified at the time of the importation if, at the time of importation,
- (a) the hazardous product or container in which the hazardous product is packaged has a label that includes all of the information elements for each category or subcategory of the hazard class in which the hazardous product is classified at the time of importation, with the exception of the significant new data; and
- (b) the supplier obtains or prepares a document that provides the significant new data and the date on which they became available.
Transfer of possession for purpose of transportation
5.13. The transfer of possession of a hazardous product that creates a bailment for the purpose of transportation or, in Quebec, the transfer of possession of a hazardous product for the purpose of transportation, without transferring ownership, and with the obligation to deliver it to the person or government that acquired possession or ownership, is exempt from the application of paragraph 13(1)(a.1) of the Act in respect of the requirement to provide, or cause to be provided, a safety data sheet to the person to whom the possession of the product is transferred for the purpose of transportation.
Definition of “transit”
5.14. (1) In this section, “transit” means, in relation to a hazardous product, its transport through Canada, after being imported and before being exported, where the place of initial loading and the final destination are outside of Canada and, while in transport, its loading, unloading, packing, unpacking or storage.
Importation — transit
(2) The importation of a hazardous product is exempt from the application of section 14 of the Act if
- (a) the hazardous product is or is intended to be in transit; and
- (b) the hazardous product is not intended to be used in a work place in Canada.
Sale — exportation
(3) The sale of a hazardous product, for the purpose of its exportation, is exempt from the application of section 13 of the Act if
- (a) the hazardous product is or is intended to be transported or, while in transport, is or is intended to be loaded, unloaded, packed, unpacked or stored, for the purpose of that sale; and
- (b) the hazardous product is not intended to be used in a work place in Canada.
PART 6
ADDITIONAL REQUIREMENTS
Communication of information elements — health professionals
6. (1) A supplier who sells or imports a hazardous product intended for use, handling or storage in a work place in Canada must provide, as soon as feasible, any information element in respect of the hazardous product that is referred to in subsection 4(1) and is in the possession of the supplier to any health professional who requests that information for the purpose of making a medical diagnosis of, or rendering medical treatment to, an individual in an emergency.
Confidentiality
(2) Any information that, by virtue of an exemption under the Hazardous Materials Information Review Act or these Regulations, is not required to be provided on the safety data sheet but has nevertheless been provided by a supplier to any health professional who requests that information for the purpose of making a medical diagnosis of, or rendering medical treatment to, an individual in a medical emergency must be kept confidential, except for the purpose for which it was provided, if the health professional has been informed by the supplier that the information is to be kept confidential.
Communication of source for toxicological data
6.1. Subject to the Hazardous Materials Information Review Act, a supplier who sells or imports a hazardous product intended for use, handling or storage in a work place in Canada must disclose, as soon as feasible, the source of information for any toxicological data used in the preparation of a safety data sheet on the request of an inspector, any person or government to whom the hazardous product is sold or any user of a hazardous product.
Bilingual safety data sheet or label
6.2. (1) The information on a safety data sheet or a label must be in both official languages of Canada.
Bilingual safety data sheet or unilingual documents
(2) The information referred to in subsection (1) may appear either on a single bilingual safety data sheet or on two separate unilingual documents that constitute one safety data sheet.
PART 7
PHYSICAL HAZARD CLASSES
SUBPART 1
EXPLOSIVES
[7.1. reserved]
SUBPART 2
FLAMMABLE GASES
Definition
Definition of “flammable gas”
7.2. In this Subpart, “flammable gas” means a gas that has a flammable range when mixed with air at 20°C and at the standard pressure of 101.3 kPa.
Classification in a Category of the Class
Exclusions
7.2.1 (1) Any product that is classified in a category of the hazard class “Flammable Aerosols” need not be classified in any category of this hazard class.
Categories
(2) A flammable gas is classified in a category of this hazard class in accordance with the following table:
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Flammable Gases — Category 1 | A gas that (a) is ignitable when mixed with air at a concentration ≤ 13.0% by volume; or (b) has a flammable range when mixed with air ≥ 12 percentage points, regardless of the lower flammable limit |
| 2. | Flammable Gases — Category 2 | A gas that is not classified in the category “Flammable Gases — Category 1” and has a flammable range when mixed with air |
Calculation method
(3) Test data have priority over data obtained using a calculation method. If a calculation method is used to establish whether a gas is classified in a category of this hazard class, the calculation method set out in the International Organization for Standardization standard ISO 10156:2010 entitled Gases and gas mixtures — Determination of fire potential and oxidizing ability for the selection of cylinder valve outlets, as amended from time to time, or any other calculation method that is a scientifically validated method, must be used.
SUBPART 3
FLAMMABLE AEROSOLS
Definitions
Definitions
7.3. The following definitions apply in this Subpart.
“flammable aerosol”
« aérosol inflammable »
“flammable aerosol” means a product that contains one or more flammable components in an aerosol dispenser and that, when dispensed, is liable to ignite, but excludes a product that contains flammable components in an aerosol dispenser at a concentration less than or equal to 1.0% and that has a heat of combustion less than 20 kJ/g.
“flammable component”
« composant inflammable »
“flammable component” means a mixture or substance that is classified in a category or subcategory of a hazard class in Subpart 2, 6 or 7 of this Part.
“foam aerosol”
« mousse d'aérosol »
“foam aerosol” means the content that is dispensed from an aerosol dispenser that has a spray distance of less than 15 cm.
“spray aerosol”
« aérosol vaporisé »
“spray aerosol” means the content that is dispensed from an aerosol dispenser that has a spray distance equal to or greater than 15 cm.
Classification in a Category of the Class
Categories
7.3.1 (1) A flammable aerosol is classified in a category of this hazard class in accordance with the following table:
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Flammable Aerosols — Category 1 | An aerosol dispenser that
|
| 2. | Flammable Aerosols — Category 2 | An aerosol dispenser that generates
|
Default category
(2) A product that contains flammable components in an aerosol dispenser for which there are no test results in accordance with subparagraph 2.1(a)(i) and referred to in subsection (1) must be classified in the category “Flammable Aerosols – Category 1”, unless the product contains flammable components at a concentration less than or equal to 1.0% and has a heat of combustion less than 20 kJ/g.
SUBPART 4
OXIDIZING GASES
Definition
Definition of “oxidizing gas”
7.4. In this Subpart, “oxidizing gas” means a gas that is liable to cause or contribute to the combustion of other material more than air does.
Classification in the Category of the Class
Category
7.4.1 An oxidizing gas is classified in the category of this hazard class in accordance with the following table:
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Oxidizing Gases — Category 1 | A gas that has an oxidizing power > 23.5% based on one of the methods set out in the International Organization for Standardization standard ISO 10156:2010 entitled Gases and gas mixtures — Determination of fire potential and oxidizing ability for the selection of cylinder valve outlets, as amended from time to time |
SUBPART 5
GASES UNDER PRESSURE
Definitions
Definitions
7.5. The following definitions apply in this Subpart.
“critical temperature”
« température critique »
“gas under pressure”
« gaz sous pression »
“critical temperature” means the temperature above which a pure gas cannot be liquefied, regardless of the degree of compression.
“gas under pressure” means a product that consists of a gas contained in a receptacle at a gauge pressure of 200 kPa or more at 20°C, or that is liquefied, or liquefied and refrigerated, but excludes any gas that has an absolute vapour pressure of not more than 300 kPa at 50°C or that is not completely gaseous at 20°C and the standard pressure of 101.3 kPa.
Classification in a Category of the Class
Categories
7.5.1 A gas under pressure is classified in a category of this hazard class in accordance with the following table:
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Gases Under Pressure — Compressed Gas | A gas that when packaged under pressure is entirely gaseous at -50°C, including all gases with a critical temperature ≤ -50°C |
| 2. | Gases Under Pressure — Liquefied Gas | A gas that when packaged under pressure is partially liquid at temperatures > -50°C |
| 3. | Gases Under Pressure — Refrigerated Liquefied Gas | A gas that when packaged is partially liquid because of its low temperature |
| 4. | Gases Under Pressure — Dissolved Gas | A gas that when packaged under pressure is dissolved in a liquid phase solvent |
SUBPART 6
FLAMMABLE LIQUIDS
Definitions
Definitions
7.6. The following definitions apply in this Subpart.
“appropriate closed-cup method”
« méthode de creuset fermé appropriée »
“appropriate closed-cup method” means the most recent versions of the methods listed in paragraph 2.6.4.2.5 of the GHS.
“flammable liquid”
« liquide inflammable »
“flammable liquid” means a liquid that has a flash point of not more than 93°C.
Classification in a Category of the Class
Exclusions
7.6.1 (1) Any product that is classified in a category of the hazard class “Flammable Aerosols” need not be classified in any category of this hazard class.
Categories
(2) A flammable liquid is classified in a category of this hazard class in accordance with the following table:
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Flammable Liquids — Category 1 | A liquid that has a flash point < 23°C and initial boiling point ≤ 35°C |
| 2. | Flammable Liquids — Category 2 | A liquid that has a flash point < 23°C and initial boiling point > 35°C |
| 3. | Flammable Liquids — Category 3 | A liquid that has a flash point ≥ 23°C and ≤ 60°C |
| 4. | Flammable Liquids — Category 4 | A liquid that has a flash point > 60°C and ≤ 93°C |
Determination of flash point — substance
(3) In the case of a liquid that is a substance, the flash point must be determined by
- (a) tests using an appropriate closed-cup method; or
- (b) use of scientific literature that reports a value obtained from an appropriate closed-cup method.
Determination of flash point — mixture
(4) In the case of a liquid that is a mixture, the flash point must be determined by
- (a) tests using an appropriate closed-cup method; or
- (b) use of an applicable calculation method under conditions for which it has been validated according to generally accepted standards of good scientific practice at the time the validation was carried out.
Lowest flash point
(5) If data obtained using the methods referred to in paragraph (4)(a) or (b) are not available, the flash point of the ingredient with the lowest flash point in the mixture must be used.
SUBPART 7
FLAMMABLE SOLIDS
Definitions
Definitions
7.7. The following definitions apply in this Subpart.
“flammable solid”
« solide inflammable »
“flammable solid” means a readily combustible solid or a solid that is liable to cause or contribute to fire through friction.
“readily combustible solid”
« solide facilement inflammable »
“readily combustible solid” means a powdered, granular or pasty mixture or substance that can be easily ignited by brief contact with an ignition source and, when ignited, has a flame that spreads rapidly.
Classification in a Category of the Class
Exclusions
7.7.1 (1) Any product that is classified in a category of the hazard class “Flammable Aerosols” need not be classified in any category of this hazard class.
Categories
(2) A flammable solid that is a readily combustible solid is classified in a category of this hazard class, based on results from testing performed in accordance with the burning rate test in subsection 33.2.1 of Part III of the Manual of Tests and Criteria, in accordance with the following table:
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Flammable Solids — Category 1 | A solid that is
|
| 2. | Flammable Solids — Category 2 | A solid that is
|
Fire through friction
(3) A flammable solid that is a solid that is liable to cause or contribute to fire through friction is classified in the category “Flammable Solids — Category 2”.
SUBPART 8
SELF-REACTIVE SUBSTANCES AND MIXTURES
Definitions
Definitions
7.8. The following definitions apply in this Subpart.
“as packaged”
« tel qu'il est emballé »
“as packaged” means packaged in the form and condition described in test series B, D, G and H of Part II of the Manual of Tests and Criteria.
“explosive properties”
« propriétés explosives »
“explosive properties” means the properties of a self-reactive substance or mixture that, in laboratory testing according to test series A, C or E of Part II of the Manual of Tests and Criteria, make the substance or mixture liable to detonate, deflagrate rapidly or show a violent effect when heated under confinement.
“self-reactive”
« autoréactif »
“self-reactive” means, in relation to a thermally unstable liquid or solid product, mixture or substance, liable to undergo a strongly exothermic decomposition, having a heat of decomposition equal to or greater than 300 J/g, even without participation of oxygen.
Classification in a Category of the Class
Exclusions
7.8.1 (1) The following need not be classified in any category of this hazard class:
- (a) mixtures or substances, or mixtures or substances as packaged, that are classified in a category of the hazard class “Organic Peroxides”; and
- (b) liquid or solid mixtures or substances that are classified in a category of the hazard class “Oxidizing Liquids” or “Oxidizing Solids”, and contain less than 5.0% of combustible organic substances.
Categories
(2) Subject to subsection (3), a self-reactive substance or mixture is classified in a category of this hazard class, based on results from testing performed in accordance with test series A to H of Part II of the Manual of Tests and Criteria, in accordance with the following table:
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Self-reactive Substances and Mixtures — Type A | A liquid or solid that, as packaged, is liable to detonate, or deflagrate rapidly |
| 2. | Self-reactive Substances and Mixtures — Type B | A liquid or solid that possesses explosive properties and, as packaged, neither detonates, nor deflagrates rapidly, but is liable to undergo a thermal explosion in that package |
| 3. | Self-reactive Substances and Mixtures — Type C | A liquid or solid that possesses explosive properties and, as packaged, neither detonates, nor deflagrates rapidly nor undergoes a thermal explosion in that package |
| 4. | Self-reactive Substances and Mixtures — Type D | In laboratory testing, a liquid or solid that
|
| 5. | Self-reactive Substances and Mixtures — Type E | In laboratory testing, a liquid or solid that neither detonates nor deflagrates and shows low or no effect when heated under confinement |
| 6. | Self-reactive Substances and Mixtures — Type F | In laboratory testing, a liquid or solid that neither detonates in the cavitated state nor deflagrates, and
|
| 7. | Self-reactive Substances and Mixtures — Type G | In laboratory testing, a liquid or solid that neither detonates in the cavitated state nor deflagrates and shows no effect when heated under confinement nor any explosive power and either
|
Exclusion after evaluation
(3) A mixture or substance with a self-accelerating decomposition temperature greater than 75°C when evaluated in a 50 kg package need not be classified in any category of this hazard class.
SUBPART 9
PYROPHORIC LIQUIDS
Definition
Definition of “pyrophoric liquid”
7.9. In this Subpart, “pyrophoric liquid” means a liquid that is liable to ignite within five minutes after coming into contact with air.
Classification in the Category of the Class
Category
7.9.1 A pyrophoric liquid is classified in the category of this hazard class, based on results from testing performed in accordance with test N.3 of subsection 33.3.1.5 of Part III of the Manual of Tests and Criteria, in accordance with the following table:
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Pyrophoric Liquids — Category 1 | A liquid that, within 5 min, either
|
SUBPART 10
PYROPHORIC SOLIDS
Definition
Definition of “pyrophoric solid”
7.10. In this Subpart, “pyrophoric solid” means a solid that is liable to ignite within five minutes after coming into contact with air.
Classification in the Category of the Class
Category
7.10.1 A pyrophoric solid is classified in the category of this hazard class, based on results from testing performed in accordance with test N.2 of subsection 33.3.1.4 of Part III of the Manual of Tests and Criteria, in accordance with the following table:
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Pyrophoric Solids — Category 1 | A solid that ignites within 5 min after coming into contact with air |
SUBPART 11
SELF-HEATING SUBSTANCES AND MIXTURES
Definition
Definition of “self-heating”
7.11. In this Subpart, “self-heating” means, in relation to a solid or liquid, liable to self-heat by reaction with air and without energy supply.
Classification in a Category of the Class
Exclusions
7.11.1 (1) The following need not be classified in any category of this hazard class:
- (a) a liquid classified in the category of the hazard class “Pyrophoric Liquids”; and
- (b) a solid classified in the category of the hazard class “Pyrophoric Solids”.
Categories
(2) Subject to subsection (3), a self-heating substance or mixture is classified in a category of this hazard class, based on results from testing performed in accordance with test N.4 of subsection 33.3.1.6 of Part III of the Manual of Tests and Criteria, in accordance with the following table:
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Self-heating Substances and Mixtures — Category 1 | A solid or liquid in respect of which a positive result is obtained in a test using a 25 mm sample cube at 140°C and the spontaneous ignition temperature of a 450 l volume of the solid or liquid is ≤ 50°C |
| 2. | Self-heating Substances and Mixtures — Category 2 | A solid or liquid in respect of which
|
Exclusion after evaluation
(3) A mixture or substance with a temperature of spontaneous combustion higher than 50°C for a volume of 27 m3 need not be classified in any category of this hazard class.
SUBPART 12
SUBSTANCES AND MIXTURES WHICH, IN CONTACT WITH WATER, EMIT FLAMMABLE GASES
General Provision
Interpretation
7.12. In this Subpart, substances and mixtures which, in contact with water, emit flammable gases are liquids and solids that, by interaction with water, are liable to become spontaneously flammable or give off flammable gases in dangerous quantities, that is, in quantities that are equal to or greater than one litre of gas per kilogram of the mixture or substance per hour.
Classification in a Category of the Class
Exclusions
7.12.1 (1) The following liquids or solids need not be classified in any category of this hazard class:
- (a) those that have a chemical structure that does not contain metals or metalloids;
- (b) those that have been shown, through accumulated experience in production or handling, not to react with water; and
- (c) those that are soluble in water to form a stable mixture.
Categories
(2) A liquid or solid which, in contact with water, emits flammable gases is classified in a category of this hazard class, based on results from testing performed in accordance with test N.5 of subsection 33.4.1.4 of Part III of the Manual of Tests and Criteria, in accordance with the following table:
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Substances and Mixtures Which, in Contact with Water, Emit Flammable Gases — Category 1 | A liquid or solid that
|
| 2. | Substances and Mixtures Which, in Contact with Water, Emit Flammable Gases — Category 2 | A liquid or solid that reacts with water at ambient temperature such that the maximum rate of evolution of flammable gas is ≥ 20 l/kg of liquid or solid per hour |
| 3. | Substances and Mixtures Which, in Contact with Water, Emit Flammable Gases — Category 3 | A liquid or solid that reacts with water at ambient temperature such that the maximum rate of evolution of flammable gas is ≥ 1 l/kg of liquid or solid per hour |
SUBPART 13
OXIDIZING LIQUIDS
Definition
Definition of “oxidizing liquid”
7.13. In this Subpart, “oxidizing liquid” means a liquid, whether or not combustible, that is liable to cause or contribute to the combustion of other material.
Classification in a Category of the Class
Exclusions
7.13.1 (1) The following liquids need not be classified in any category of this hazard class:
- (a) any organic liquid that does not contain oxygen, fluorine or chlorine;
- (b) any organic liquid that contains oxygen, fluorine or chlorine if those elements are chemically bonded only to carbon or hydrogen; and
- (c) any inorganic liquid that does not contain oxygen or halogens.
Categories
(2) An oxidizing liquid is classified in a category of this hazard class, based on results from testing performed in accordance with test O.2 of subsection 34.4.2 of Part III of the Manual of Tests and Criteria, in accordance with the following table:
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Oxidizing Liquids — Category 1 | A liquid that, when tested in a 1:1 mixture, by mass, with cellulose, spontaneously ignites, or exhibits a mean pressure rise time < the mean pressure rise time of a 1:1 mixture, by mass, of 50.0% perchloric acid and cellulose |
| 2. | Oxidizing Liquids — Category 2 | A liquid that, when tested in a 1:1 mixture, by mass, with cellulose, exhibits a mean pressure rise time ≤ the mean pressure rise time of a 1:1 mixture, by mass, of 40.0% aqueous sodium chlorate solution and cellulose |
| 3. | Oxidizing Liquids — Category 3 | A liquid that, when tested in a 1:1 mixture, by mass, with cellulose, exhibits a mean pressure rise time ≤ the mean pressure rise time of a 1:1 mixture, by mass, of 65.0% aqueous nitric acid and cellulose |
SUBPART 14
OXIDIZING SOLIDS
Definition
Definition of “oxidizing solid”
7.14. In this Subpart, “oxidizing solid” means a solid, whether or not combustible, that is liable to cause or contribute to the combustion of other material.
Classification in a Category of the Class
Exclusions
7.14.1 (1) The following solids need not be classified in any category of this hazard class:
- (a) any organic solid that does not contain oxygen, fluorine or chlorine;
- (b) any organic solid that contains oxygen, fluorine or chlorine if those elements are chemically bonded only to carbon or hydrogen; and
- (c) any inorganic solid that does not contain oxygen or halogens.
Categories
(2) An oxidizing solid is classified in a category of this hazard class, based on results from testing performed in accordance with test O.1 of subsection 34.4.1 of Part III of the Manual of Tests and Criteria, in accordance with the following table:
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Oxidizing Solids — Category 1 | A solid that, when tested in a 4:1 or 1:1 mixture, by mass, with cellulose, exhibits a mean burning time < the mean burning time of a 3:2 mixture, by mass, of potassium bromate and cellulose |
| 2. | Oxidizing Solids — Category 2 | A solid that, when tested in a 4:1 or 1:1 mixture, by mass, with cellulose, exhibits a mean burning time ≤ the mean burning time of a 2:3 mixture, by mass, of potassium bromate and cellulose |
| 3. | Oxidizing Solids — Category 3 | A solid that, when tested in a 4:1 or 1:1 mixture, by mass, with cellulose, exhibits a mean burning time ≤ the mean burning time of a 3:7 mixture, by mass, of potassium bromate and cellulose |
SUBPART 15
ORGANIC PEROXIDES
Definitions
Definitions
7.15. The following definitions apply in this Subpart.
“as packaged”
« tel qu'il est emballé »
“as packaged” means packaged in the form and condition described in test series B, D, G and H of Part II of the Manual of Tests and Criteria.
“explosive properties”
« propriétés explosives »
“explosive properties” means the properties of an organic peroxide that, in laboratory testing according to test series A, C or E of Part II of the Manual of Tests and Criteria, make the liquid or solid liable to detonate, deflagrate rapidly or show a violent effect when heated under confinement.
“organic peroxide”
« peroxyde organique »
“organic peroxide” means an organic liquid or solid that contains the bivalent -O-O- structure.
Classification in a Category of the Class
Exclusions
7.15.1 (1) An organic peroxide that contains any of the following need not be classified in any category of this hazard class:
- (a) not more than 1.0% available oxygen from the organic peroxides when containing not more than 1.0% hydrogen peroxide; or
- (b) not more than 0.5% available oxygen from the organic peroxides when containing more than 1.0% but not more than 7.0% hydrogen peroxide.
Available oxygen content
(2) The available oxygen content, in percent, of an organic peroxide mixture referred to in paragraph (1)(a) or (b) is determined by the formula:
![]()
where
ni is the number of peroxygen groups per molecule of organic peroxide i;
ci is the concentration (mass %) of organic peroxide i; and
mi is the molecular mass of organic peroxide i.
Categories
(3) An organic peroxide is classified in a category of this hazard class, based on results from testing performed in accordance with test series A to H of Part II of the Manual of Tests and Criteria, in accordance with the following table:
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Organic Peroxides — Type A | A liquid or solid that, as packaged, is liable to detonate, or deflagrate rapidly |
| 2. | Organic Peroxides — Type B | A liquid or solid that possesses explosive properties and, as packaged, neither detonates, nor deflagrates rapidly, but is liable to undergo a thermal explosion in that package |
| 3. | Organic Peroxides — Type C | A liquid or solid that possesses explosive properties and, as packaged, neither detonates, nor deflagrates rapidly, nor undergoes a thermal explosion in that package |
| 4. | Organic Peroxides — Type D | In laboratory testing, a liquid or solid that
|
| 5. | Organic Peroxides — Type E | In laboratory testing, a liquid or solid that neither detonates nor deflagrates, and shows low or no effect when heated under confinement |
| 6. | Organic Peroxides — Type F | In laboratory testing, a liquid or solid that neither detonates in the cavitated state nor deflagrates and
|
| 7. | Organic Peroxides — Type G | In laboratory testing, a liquid or solid that neither detonates in the cavitated state nor deflagrates, and shows no effect when heated under confinement nor any explosive power, and either
|
Mixtures
(4) A mixture of organic peroxides must be classified in the same category as the most hazardous organic peroxide in the mixture, unless the self-accelerating decomposition temperature of the mixture results in the mixture being classified in a category that represents a more severe hazard.
SUBPART 16
CORROSIVE TO METALS
Definition
Definition of “corrosive to metals”
7.16. In this Subpart, “corrosive to metals” means, in relation to a mixture or substance, liable to damage or destroy metal by chemical action.
Classification in the Category of the Class
Category
7.16.1 A mixture or substance that is corrosive to metals is classified in the category of this hazard class, based on results from testing performed in accordance with subsection 37.4 of Part III of the Manual of Tests and Criteria, in accordance with the following table:
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Corrosive to Metals — Category 1 | A mixture or substance that has a corrosion rate on either steel or aluminium surfaces that is > 6.25 mm per year at a test temperature of 55°C |
SUBPART 17
COMBUSTIBLE DUSTS
Definition
Definition of “combustible dust”
7.17. In this Subpart, “combustible dust” means a mixture or substance that is in the form of a powder that, upon ignition, is liable to catch fire or explode when dispersed in air or an other oxidizing medium.
Classification in the Category of the Class
Category
7.17.1 A combustible dust is classified in the category of this hazard class in accordance with the following table:
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Combustible Dusts — Category 1 | A mixture or substance that
|
SUBPART 18
SIMPLE ASPHYXIANTS
Definition
Definition of “simple asphyxiant”
7.18. In this Subpart, “simple asphyxiant” means any gas that is liable to cause asphyxiation by the displacement of air.
Classification in the Category of the Class
Category
7.18.1 A simple asphyxiant is classified in the category of this hazard class in accordance with the following table:
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Simple Asphyxiants — Category 1 | A gas that is a simple asphyxiant |
SUBPART 19
PYROPHORIC GASES
Definition
Definition of “pyrophoric gas”
7.19. In this Subpart, “pyrophoric gas” means any mixture or substance in a gaseous state that is liable to ignite spontaneously in air at a temperature of 54.4°C or less.
Classification in the Category of the Class
Category
7.19.1 A pyrophoric gas is classified in the category of this hazard class in accordance with the following table:
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Pyrophoric Gases — Category 1 | A gas that is a pyrophoric gas |
SUBPART 20
PHYSICAL HAZARDS NOT OTHERWISE CLASSIFIED
Definition
Definition of “physical hazard not otherwise classified”
7.20. In this Subpart, “physical hazard not otherwise classified” means a physical hazard presented by a product, mixture, material or substance that is different from any other physical hazard addressed by any other Subpart in this Part, and that has the characteristic of occurring by chemical reaction and resulting in the death or serious injury of a person at the time the reaction occurs.
Classification in the Category of the Class
Category
7.20.1 A product, mixture, material or substance is classified in the category of this hazard class in accordance with the following table:
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Physical Hazards Not Otherwise Classified — Category 1 | A product, mixture, material or substance that presents a physical hazard not otherwise classified |
PART 8
HEALTH HAZARD CLASSES
SUBPART 1
ACUTE TOXICITY
Definitions
Definitions
8.1. The following definitions apply in this Subpart.
“acute toxicant”
« toxique aigu »
“acute toxicant” means a mixture or substance that is liable to cause acute toxicity, or a mixture or substance that, upon contact with water, releases a gaseous substance that is liable to cause acute toxicity.
“acute toxicity”
« toxicité aiguë »
“acute toxicity” refers to adverse effects occurring following
- (a) oral or dermal administration of a single dose of a mixture or substance, or multiple doses given within 24 hours; or
- (b) an inhalation exposure to a mixture or substance of four hours or of a duration that is converted to four hours in accordance with subsection 8.1.1(4).
“dust”
« poussières »
“dust” means solid particles that are suspended in a gas, usually air.
“mist”
« brouillard »
“mist” means liquid droplets suspended in the air.
Classification in a Category of the Class
Classification of Substances
LD50 or LC50 — associated range
8.1.1 (1) An acute toxicant that is a substance is classified, with respect to each applicable route of exposure, in a category of this hazard class in accordance with the tables to subsection (3) if it has an LD50 by the oral or dermal exposure route, or an LC50 by the inhalation exposure route, that falls into one of the ranges indicated in the applicable table to that subsection.
Contact with water — gaseous substance
(2) In addition to subsection (1), an acute toxicant that is a substance is classified with respect to the inhalation route of exposure in a category of this hazard class in accordance with Table 3 to subsection (3) if upon contact with water, it releases a gaseous substance that has an LC50 that falls into one of the ranges indicated in that table, unless that substance is already classified in a category of this hazard class for the inhalation route of exposure that represents a more severe hazard.
LD50 or LC50 not available
(3) If an LD50 by the oral or dermal exposure route or an LC50 by the inhalation exposure route is not available, an acute toxicity point estimate must be established in accordance with the table to section 8.1.7, and the acute toxicant must be classified based on that acute toxicity point estimate, with respect to each applicable route of exposure, in a category of this hazard class in accordance with the following tables:
TABLE 1
| Item | Column 1 Category |
Column 2 Ranges for LD50 or for Acute Toxicity Point Estimates (mg/kg body weight) |
|---|---|---|
| 1. | Acute Toxicity (Oral) — Category 1 | ≤ 5 |
| 2. | Acute Toxicity (Oral) — Category 2 | > 5 and ≤ 50 |
| 3. | Acute Toxicity (Oral) — Category 3 | > 50 and ≤ 300 |
| 4. | Acute Toxicity (Oral) — Category 4 | > 300 and ≤ 2000 |
TABLE 2
| Item | Column 1 Category |
Column 2 Ranges for LD50 or for Acute Toxicity Point Estimates (mg/kg body weight ) |
|---|---|---|
| 1. | Acute Toxicity (Dermal) — Category 1 | ≤ 50 |
| 2. | Acute Toxicity (Dermal) — Category 2 | > 50 and ≤ 200 |
| 3. | Acute Toxicity (Dermal) — Category 3 | > 200 and ≤ 1000 |
| 4. | Acute Toxicity (Dermal) — Category 4 | > 1000 and ≤ 2000 |
TABLE 3
| Item | Column 1 Category |
Column 2 Gases (ppmV) |
Column 3 Ranges for LC50 or for Acute Toxicity Point Estimates Vapours (mg/l) |
Column 4 Dusts and Mists (mg/l) |
|---|---|---|---|---|
| 1. | Acute Toxicity (Inhalation) — Category 1 | ≤ 100 | ≤ 0.5 | ≤ 0.05 |
| 2. | Acute Toxicity (Inhalation) — Category 2 | > 100 and ≤ 500 | > 0.5 and ≤ 2 | > 0.05 and ≤ 0.5 |
| 3. | Acute Toxicity (Inhalation) — Category 3 | > 500 and ≤ 2500 | > 2 and ≤ 10 | > 0.5 and ≤ 1 |
| 4. | Acute Toxicity (Inhalation) — Category 4 | > 2500 and ≤ 20 000 | > 10 and ≤ 20 | > 1 and ≤ 5 |
One-hour exposure period
(4) For the purposes of Table 3 to subsection (3), the LC50 is based on a four-hour exposure period. If existing acute inhalation toxicity data have been generated according to a one-hour exposure period, the LC50 for gases and vapours must be divided by two, and the LC50 for dusts and mists must be divided by four.
Classification of Mixtures
Order of provisions
8.1.2 (1) The classification of a mixture as an acute toxicant in a category of this hazard class must proceed in accordance with the order of sections 8.1.3 to 8.1.6.
Concentrations for the purpose of classification
(2) Only ingredients present at concentrations equal to or greater than the concentration limit of 1.0% — w/w for solids, liquids, dusts, mists and vapours and v/v for gases — must be considered for the purpose of classification.
Data available for mixture as a whole
8.1.3 If data of the types referred to in subparagraphs 2.1(a)(i) to (iv) are available for the mixture as a whole, the mixture must be classified as an acute toxicant in accordance with section 8.1.1.
Data available for use of bridging principles
8.1.4 If data are available to enable the characterization of the mixture as an acute toxicant, in accordance with the bridging principles referred to in subsections 2.3(3) to (8), the mixture must be classified in a category of this hazard class in accordance with those subsections.
Data available for all ingredients
8.1.5 If data are available for all ingredients in the mixture, the mixture must be classified as an acute toxicant in accordance with section 8.1.1 using the ATE of the mixture that is determined in respect of each applicable route of exposure by the formula
where
ATEmix is the ATE of the mixture determined using this formula;
Ci is the concentration of ingredient i;
n is the number of ingredients and i is running from 1 to n;
ATEi is the ATE of ingredient i, which is either
- (a) the LD50 or the LC50 based on or converted to a four-hour exposure period, for i, or
- (b) if the LD50 or the LC50 is unavailable, the acute toxicity point estimate established for i in accordance with the table to section 8.1.7; and
i is each ingredient in the mixture with
- (a) an ATE within the ranges set out in the applicable table to subsection 8.1.1(3),
- (b) an oral or dermal LD50 greater than 2000 mg/kg body weight but less than or equal to 5000 mg/kg body weight, or
- (c) an LC50 based on or converted to a four-hour exposure period within a range having an amplitude comparable to the one in paragraph (b).
Data not available for all ingredients
8.1.6 If the ATE is not available for one or more ingredients of the mixture, the mixture must be classified as an acute toxicant in accordance with section 8.1.1 using the ATE of the mixture that is determined in respect of each applicable route of exposure according to the following:
- (a) if data permit the ATE to be estimated for each of those ingredients in accordance with established scientific principles, the formula in section 8.1.5 must be used;
- (b) if data do not permit the ATE to be estimated for an ingredient in accordance with established scientific principles, and the concentration of the ingredient in the mixture is equal to or greater than the concentration limit of 1.0%, the mixture is classified based only on the ingredients having an ATE, such that
- (i) if the total concentration of all ingredients with unknown acute toxicity is less than or equal to 10.0% of the mixture, the formula in section 8.1.5 must be used, or
- (ii) if the total concentration of all ingredients with unknown acute toxicity is greater than 10.0% of the mixture, the following formula must be used:
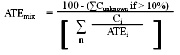
where
ATEmix is the ATE of the mixture determined using this formula,
Ci is the concentration of ingredient i,
Cunknown is the concentration of ingredients i with unknown ATE values,
n is the number of ingredients and i is running from 1 to n,
ATEi is the ATE of ingredient i, which is either
- (a) the LD50 or the LC50 based on or converted to a four-hour exposure period, for i, or
- (b) if the LD50 or the LC50 is unavailable, the acute toxicity point estimate established for i in accordance with the table to section 8.1.7, and
i is each ingredient in the mixture with
- (a) an ATE within the ranges set out in the applicable table to subsection 8.1.1(3),
- (b) an oral or dermal LD50 greater than 2000 mg/kg body weight but less than or equal to 5000 mg/kg body weight, or
- (c) an LC50 based on or converted to a four-hour exposure period within a range having an amplitude comparable to the one in paragraph (b).
Conversion from range to point estimate
8.1.7 If a formula in section 8.1.5 or 8.1.6 is used, an acute toxicity point estimate must be determined, in accordance with the following table, for each ingredient for which only that ingredient's classification category or experimentally obtained acute toxicity range is available:
| Item | Column 1 Exposure Routes |
Column 2 Classification Category and Associated Experimentally Obtained Acute Toxicity Range Minimum and Maximum Values |
Column 3 Converted Acute Toxicity Point Estimate |
|---|---|---|---|
| 1. | Oral (mg/kg body weight ) | 0 < Category 1 ≤ 5 5 < Category 2 ≤ 50 50 < Category 3 ≤ 300 300 < Category 4 ≤ 2000 | 0.5 5 100 500 |
| 2. | Dermal (mg/kg body weight) | 0 < Category 1 ≤ 50 50 < Category 2 ≤ 200 200 < Category 3 ≤ 1000 1000 < Category 4 ≤ 2000 | 5 50 300 1100 |
| 3. | Inhalation (gases) (ppmV) | 0 < Category 1 ≤ 100 100 < Category 2 ≤ 500 500 < Category 3 ≤ 2500 2500 < Category 4 ≤ 20 000 | 10 100 700 4500 |
| 4. | Inhalation (vapours) (mg/l) | 0 < Category 1 ≤ 0.5 0.5 < Category 2 ≤ 2.0 2.0 < Category 3 ≤ 10.0 10.0 < Category 4 ≤ 20.0 | 0.05 0.5 3 11 |
| 5. | Inhalation (dust/mist) (mg/l) | 0 < Category 1 ≤ 0.05 0.05 < Category 2 ≤ 0.5 0.5 < Category 3 ≤ 1.0 1.0 < Category 4 ≤ 5.0 | 0.005 0.05 0.5 1.5 |
SUBPART 2
SKIN CORROSION/IRRITATION
Definitions
Definitions
8.2. The following definitions apply in this Subpart.
“skin corrosion”
« corrosion cutanée »
“skin corrosion” means the production of irreversible damage to the skin, namely, visible necrosis through the epidermis and into the dermis, and includes ulcers, bleeding, bloody scabs and, within a 14-day observation period, discoloration due to blanching of the skin, complete areas of alopecia, and scars.
“skin-corrosive”
« corrosif pour la peau »
“skin-corrosive” means, in relation to a mixture or substance, liable to cause skin corrosion.
“skin-irritant”
« irritant pour la peau »
“skin-irritant” means, in relation to a mixture or substance, liable to cause skin irritation.
“skin irritation”
« irritation cutanée »
“skin irritation” means the production of reversible damage to the skin.
Classification in a Category or Subcategory of the Class
Classification of Substances
Order of provisions
8.2.1 The classification of a skin-corrosive substance or a skin-irritant substance in a category or subcategory of this hazard class must proceed in accordance with the order of sections 8.2.2 to 8.2.7, unless, after applying subsections 8.2.2(1) to (3), the substance is not classified further to subsection 8.2.2(4).
Human data — skin corrosion
8.2.2 (1) A substance for which human data demonstrate that it is a skin-corrosive substance is classified in the category “Skin Corrosion — Category 1”.
Animal data — skin corrosion
(2) A substance for which purposely generated animal data demonstrate that it is a skin-corrosive substance is classified in the category “Skin Corrosion — Category 1” and is, if the applicable data are available, further classified in accordance with the following table:
| Item | Column 1 Subcategory |
Column 2 Criteria |
|---|---|---|
| 1. | Skin Corrosion — Category 1A | A substance that, according to animal data acquired from a scientifically validated method, produces irreversible damage to the skin after an exposure of three minutes or less, and within one hour of observation, in at least one of three animals |
| 2. | Skin Corrosion — Category 1B | A substance that, according to animal data acquired from a scientifically validated method, produces irreversible damage to the skin after an exposure of more than three minutes and up to and including one hour, and within 14 days of observation, in at least one of three animals |
| 3. | Skin Corrosion — Category 1C | A substance that, according to animal data acquired from a scientifically validated method, produces irreversible damage to the skin after an exposure of more than one hour and up to and including four hours, and within 14 days of observation, in at least one of three animals |
Human or animal data — skin irritation
(3) A substance for which there are human data or purposely generated animal data with respect to skin irritation is classified in the category “Skin Irritation — Category 2” in accordance with the following table:
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Skin Irritation — Category 2 | A substance
|
No classification
(4) A substance that meets the following conditions need not be classified in any category or subcategory of this hazard class:
- (a) there are human data or purposely generated animal data on the substance, acquired from a scientifically validated method, with respect to skin corrosion or skin irritation;
- (b) the substance is not classified further to subsection (1), (2) or (3); and
- (c) the data referred to in paragraph (a) demonstrate that it is neither a skin-corrosive substance nor a skin-irritant substance.
Other skin data from animals
8.2.3 A substance for which there are animal data on dermal exposure, acquired from a scientifically validated method, that have not been purposely generated and that demonstrate that the substance is skin-corrosive or skin-irritant is classified, respectively, in the category “Skin Corrosion — Category 1” or the category “Skin Irritation — Category 2”.
In vitro or ex vivo data
8.2.4 A substance for which the data, in vitro or ex vivo, acquired from a scientifically validated method for the evaluation of skin corrosion or skin irritation demonstrate that the substance is skincorrosive or skin-irritant is classified, respectively, in the category “Skin Corrosion — Category 1” or the category “Skin Irritation — Category 2”.
pH
8.2.5 A substance for which the pH is less than or equal to two or equal to or greater than 11.5 is classified in the category “Skin Corrosion — Category 1”, unless an assessment of alkali or acid reserve performed in accordance with established scientific principles supports the conclusion that it need not be classified as a skin-corrosive substance on the basis of its pH.
Structure-activity relationship — skin corrosion
8.2.6 (1) A substance for which a structureactivity relationship, established in accordance with established scientific principles, supports the conclusion that the substance must be classified in the category “Skin Corrosion — Category 1” is classified in that category.
Structure-activity relationship — skin irritation
(2) A substance for which a structure-activity relationship, established in accordance with established scientific principles, supports the conclusion that the substance must be classified in the category “Skin Irritation — Category 2” is classified in that category.
Totality of available data
8.2.7 A substance for which an evaluation of the totality of available data, performed in accordance with established scientific principles, supports the conclusion that the substance is skin-corrosive or skin-irritant is classified, respectively, in the category “Skin Corrosion — Category 1” or the category “Skin Irritation — Category 2”.
Classification of Mixtures
Order of provisions
8.2.8 The classification of a mixture as skincorrosive or as skin-irritant in a category or subcategory of this hazard class must proceed in accordance with the order of sections 8.2.9 to 8.2.11.
Data available for mixture as a whole
8.2.9 (1) If data of the types referred to in subparagraphs 2.1(a)(i) to (iv) are available for the mixture as a whole, the mixture must be classified in accordance with the order of sections 8.2.2 to 8.2.7, unless under subsection 8.2.2(4) the mixture need not be classified.
Data available for mixture as a whole — sections 8.2.10 and 8.2.11
(2) If data of the types referred to in subparagraphs 2.1(a)(i) to (iv) are available for the mixture as a whole, but the mixture cannot be classified further to subsections 8.2.2(1) to (3) or sections 8.2.3 to 8.2.7, its classification in a category or subcategory of this hazard class must proceed in accordance with the order of sections 8.2.10 and 8.2.11.
Data available for use of bridging principles
8.2.10 If data are available to enable the characterization of the mixture as a skin-corrosive mixture or a skin-irritant mixture, in accordance with the bridging principles referred to in subsections 2.3(3) to (8), the mixture must be classified in a category or subcategory of this hazard class in accordance with those subsections.
Data available for ingredients
8.2.11 (1) Subject to subsection (3), a mixture that contains one or more ingredients that are classified in the category “Skin Corrosion — Category 1” or the category “Skin Irritation — Category 2” is classified in a category or subcategory of this hazard class in accordance with subsection (2), subject to the following:
- (a) ingredients that are classified in the category “Skin Corrosion — Category 1” or the category “Skin Irritation — Category 2” and are present in the mixture at a concentration equal to or greater than the concentration limit of 1.0% must be included in the calculation of the sum of concentrations of ingredients; and
- (b) ingredients that are classified in the category “Skin Corrosion — Category 1” or the category “Skin Irritation — Category 2” and are present in the mixture at a concentration of less than the concentration limit of 1.0% must be included in the calculation of the sum of concentrations of ingredients only if there is evidence that, at the concentration at which they are present, the ingredients are skin-corrosive substances or skin-irritant substances.
Classification — mixture
(2) A mixture is classified in a category of this hazard class in accordance with the following:
- (a) if the sum of concentrations of ingredients classified in the category “Skin Corrosion — Category 1” is equal to or greater than 5.0%, the mixture is classified in the category “Skin Corrosion — Category 1”;
- (b) if the sum of concentrations of ingredients classified in the category “Skin Corrosion — Category 1” is equal to or greater than 1.0% but less than 5.0%, the mixture is classified in the category “Skin Irritation — Category 2”;
- (c) if the sum of concentrations of ingredients classified in the category “Skin Irritation — Category 2” is equal to or greater than 10.0%, the mixture is classified in the category “Skin Irritation — Category 2”; or
- (d) if the sum of the results of the following subparagraphs is equal to or greater than 10.0%, the mixture is classified in the category “Skin Irritation — Category 2”:
- (i) 10 times the sum of concentrations of ingredients classified in the category “Skin Corrosion — Category 1”, and
- (ii) the sum of concentrations of ingredients classified in the category “Skin Irritation — Category 2”.
Mixtures containing particular ingredients
(3) A mixture is classified in a category of this hazard class in accordance with the following table if it contains one or more substances such as acids, bases, inorganic salts, aldehydes, phenols or surfactants which could be corrosive or irritant at concentrations below the concentration limits set out in subsection (2) and at least one ingredient with a concentration that is above the concentration limits set out below:
| Item | Column 1 Category |
Column 2 Ingredient |
Column 3 Concentration Limits |
|---|---|---|---|
| 1. | Skin Corrosion — Category 1 | Acid with pH ≤ 2 | ≥ 1.0% |
| 2. | Skin Corrosion — Category 1 | Base with pH ≥ 11.5 | ≥ 1.0% |
| 3. | Skin Corrosion — Category 1 | Other ingredients classified in the category “Skin Corrosion — Category 1” | ≥ 1.0% |
| 4. | Skin Irritation — Category 2 | Other ingredients classified in the category “Skin Irritation — Category 2”, including acids and bases | ≥ 3.0% |
SUBPART 3
SERIOUS EYE DAMAGE/EYE IRRITATION
Definitions
Definitions
8.3. The following definitions apply in this Subpart.
“eye irritation” « irritation oculaire »
“eye irritation” means the production of changes in the eye that are fully reversible within an observation period of 21 days.
“serious eye damage” « lésion oculaire grave »
“serious eye damage” means the production of tissue damage in the eye or serious physical decay of vision
- (a) for which data demonstrate that it is irreversible; or
- (b) that is not fully reversible within an observation period of 21 days.
Classification in a Category or Subcategory of the Class
Classification of Substances
Order of provisions
8.3.1 The classification of a substance that causes serious eye damage or eye irritation in a category or subcategory of this hazard class must proceed in accordance with the order of sections 8.3.2 to 8.3.7, unless, after applying subsections 8.3.2(1) to (4), the substance is not classified further to subsection 8.3.2(5).
Human or animal data — serious eye damage
8.3.2 (1) A substance for which there are human data or purposely generated animal data with respect to serious eye damage is classified in the category of this hazard class in accordance with the following table:
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Serious Eye Damage — Category 1 | A substance
|
Human data — eye irritation
(2) A substance for which human data demonstrate that it causes eye irritation is classified in the category “Eye Irritation — Category 2”.
Animal data — eye irritation
(3) A substance for which purposely generated animal data demonstrate that it causes eye irritation is classified in the category “Eye Irritation — Category 2” and is, if the applicable data are available, further classified in the appropriate subcategory in accordance with the following table:
| Item | Column 1 Category |
Column 2 Subcategory |
Column 3 Criteria |
|---|---|---|---|
| 1. | Eye Irritation — Category 2 | Eye Irritation — Category 2A | A substance that is not classified in the category “Serious Eye Damage — Category 1” and in respect of which animal data acquired from tests performed in accordance with the OECD Guideline for the Testing of Chemicals, No. 405, entitled Acute Eye Irritation/Corrosion, as amended from time to time, demonstrate in at least two of three animals a positive response that fully reverses within an observation period of more than seven days but not more than 21 days, and the mean score calculated following grading at 24, 48 and 72 hours afterinstillation of the substance, in relation to the positive result, is
|
| 2. | Eye Irritation — Category 2 | Eye Irritation — Category 2B | A substance that is not classified in the category “Serious Eye Damage — Category 1” and in respect of which animal data acquired from tests performed in accordance with the OECD Guideline for the Testing of Chemicals, No. 405, entitled Acute Eye Irritation/Corrosion, as amended from time to time, demonstrate in at least two of three animals a positive response that fully reverses within an observation period of seven days, and the mean score calculated following grading at 24, 48 and 72 hours after instillation of the substance, in relation to the positive result, is
|
Skin corrosion data
(4) A substance that is classified in the category “Skin Corrosion — Category 1” in accordance with subsections 8.2.2(1) and (2) is also classified in the category “Serious Eye Damage — Category 1” of this hazard class.
No classification
(5) A substance that meets the following conditions need not be classified in any category of this hazard class:
- (a) there are human data or purposely generated animal data on the substance, acquired from a scientifically validated method, with respect to serious eye damage or eye irritation;
- (b) the substance is not classified further to subsections (1) to (4); and
- (c) the data referred to in paragraph (a) demonstrate that the substance does not cause serious eye damage or eye irritation.
Other animal data — eye or skin exposure
8.3.3 A substance for which there are animal data on eye exposure that demonstrate, or animal data on skin exposure that support the conclusion, that the substance causes serious eye damage or eye irritation is classified in the category “Serious Eye Damage — Category 1” or the category “Eye Irritation — Category 2”, and, in the latter case, if the applicable data are available, the substance is further classified in the subcategory “Eye Irritation — Category 2A” or in the subcategory “Eye Irritation — Category 2B”.
In vitro or ex vivo data — serious eye damage
8.3.4 (1) A substance for which the data, in vitro or ex vivo, acquired from a scientifically validated method for the evaluation of serious eye damage demonstrate that the substance causes serious eye damage is classified in the category “Serious Eye Damage — Category 1”.
In vitro or ex vivo data — eye irritation
(2) A substance for which the data, in vitro or ex vivo, acquired from a scientifically validated method for the evaluation of eye irritation demonstrate that the substance causes eye irritation is classified in the category “Eye Irritation — Category 2” and, if the applicable data are available, the substance is further classified in the subcategory “Eye Irritation — Category 2A” or in the subcategory “Eye Irritation — Category 2B”.
pH
8.3.5 A substance for which the pH is less than or equal to two or equal to or greater than 11.5 is classified in the category “Serious Eye Damage — Category 1”, unless an assessment of alkali or acid reserve performed in accordance with established scientific principles supports the conclusion that it need not be classified as a substance that causes serious eye damage on the basis of its pH.
Structureactivity relationship — serious eye damage
8.3.6 (1) A substance for which a structureactivity relationship, established in accordance with established scientific principles, supports the conclusion that the substance must be classified in the category “Serious Eye Damage — Category 1” is classified in that category.
Structureactivity relationship — eye irritation
(2) A substance for which a structure-activity relationship, established in accordance with established scientific principles, supports the conclusion that the substance must be classified in the category “Eye Irritation — Category 2” is classified in that category and, if the applicable data are available, the substance is further classified in the subcategory “Eye Irritation — Category 2A” or in the subcategory “Eye Irritation — Category 2B”.
Totality of available data
8.3.7 A substance for which an evaluation of the totality of available data, performed in accordance with established scientific principles, supports the conclusion that the substance causes serious eye damage or eye irritation is classified in the category “Serious Eye Damage — Category 1” or the category “Eye Irritation — Category 2”and, in the latter case, if the applicable data are available, the substance is further classified in the subcategory “Eye Irritation — Category 2A” or in the subcategory “Eye Irritation — Category 2B”.
Classification of Mixtures
Order of provisions
8.3.8 The classification of a mixture as a mixture that causes serious eye damage or eye irritation in a category of this hazard class must proceed in accordance with the order of sections 8.3.9 to 8.3.11.
Data available for mixture as a whole
8.3.9 (1) If data of the types referred to in subparagraphs 2.1(a)(i) to (iv) are available for the mixture as a whole, the mixture must be classified in accordance with the order of sections 8.3.2 to 8.3.7 unless, under subsection 8.3.2(5), the mixture need not be classified.
Data available for mixture as a whole — sections 8.3.10 and 8.3.11
(2) If data of the types referred to in subparagraphs 2.1(a)(i) to (iv) are available for the mixture as a whole, but the mixture cannot be classified further to subsections 8.3.2(1) to (4) or sections 8.3.3 to 8.3.7, its classification in a category or subcategory of this hazard class must proceed in accordance with the order of sections 8.3.10 and 8.3.11.
Data available for use of bridging principles
8.3.10 If data are available to enable the characterization of the mixture as a mixture that causes serious eye damage or eye irritation, in accordance with the bridging principles referred to in subsections 2.3(3) to (8), the mixture must be classified in a category or subcategory of this hazard class in accordance with those subsections.
Data available for ingredients
8.3.11 (1) Subject to subsection (3), a mixture that contains one or more ingredients that are classified in the category “Serious Eye Damage — Category 1” or the category “Eye Irritation — Category 2” is classified in a category of this hazard class in accordance with subsection (2), subject to the following:
- (a) ingredients that are classified in the category “Serious Eye Damage — Category 1” or the category “Eye Irritation — Category 2” and are present in the mixture at a concentration equal to or greater than the concentration limit of 1.0% must be included in the calculation of the sum of concentrations of ingredients; and
- (b) ingredients that are classified in the category “Serious Eye Damage — Category 1” or the category “Eye Irritation — Category 2” and are present in the mixture at a concentration of less than the concentration limit of 1.0% must be included in the calculation of the sum of concentrations of ingredients only if there is evidence that, at the concentration at which they are present, the ingredients are substances that cause serious eye damage or eye irritation.
Classification — mixture
(2) A mixture is classified in a category of this hazard class in accordance with the following:
- (a) if the sum of concentrations of ingredients classified in the categories “Serious Eye Damage — Category 1” and “Skin Corrosion — Category 1” is equal to or greater than 3.0%, the mixture is classified in the category “Serious Eye Damage — Category 1”;
- (b) if the sum of concentrations of ingredients classified in the categories “Serious Eye Damage — Category 1” and “Skin Corrosion — Category 1” is equal to or greater than 1.0% but less than 3.0%, the mixture is classified in the category “Eye Irritation — Category 2”;
- (c) if the sum of concentrations of ingredients classified in the category “Eye Irritation — Category 2” is equal to or greater than 10.0%, the mixture is classified in the category “Eye Irritation — Category 2”; or
- (d) if the sum of the results of the following subparagraphs is equal to or greater than 10.0%, the mixture is classified in the category “Eye Irritation — Category 2”:
- (i) 10 times the total of the sum of concentrations of ingredients classified in the category “Serious Eye Damage — Category 1” and the sum of concentrations of ingredients classified in the category “Skin Corrosion — Category 1”, and
- (ii) the sum of concentrations of ingredients classified in the category “Eye Irritation — Category 2”.
Mixtures containing particular ingredients
(3) A mixture is classified in a category of this hazard class in accordance with the following table if the mixture contains one or more substances such as acids, bases, inorganic salts, aldehydes, phenols or surfactants which could cause serious eye damage or eye irritation at concentrations below the concentration limits set out in subsection (2) and at least one ingredient with a concentration that is above the concentration limits set out below:
| Item | Column 1 Category |
Column 2 Ingredient |
Column 3 Concentration Limits |
|---|---|---|---|
| 1. | Serious Eye Damage — Category 1 | Acid with pH ≤ 2 | ≥ 1.0% |
| 2. | Serious Eye Damage — Category 1 | Base with pH ≥ 11.5 | ≥ 1.0% |
| 3. | Serious Eye Damage — Category 1 | Other ingredients classified in the category “Serious Eye Damage — Category 1” | ≥ 1.0% |
| 4. | Eye Irritation — Category 2 | Other ingredients classified in the category “Eye Irritation — Category 2”, including acids and bases | ≥ 3.0% |
SUBPART 4
RESPIRATORY OR SKIN SENSITIZATION
Definitions
Definitions
8.4. The following definitions apply in this Subpart.
“respiratory sensitization”
« sensibilisation respiratoire »
“respiratory sensitization” means the production of hypersensitivity of the airways following inhalation.
“respiratory sensitizer”
« sensibilisant respiratoire »
“respiratory sensitizer” means a mixture or substance that is liable to lead to hypersensitivity of the airways following inhalation.
“skin sensitization”
« sensibilisation cutanée »
“skin sensitization” means the production of an allergic response following skin contact.
“skin sensitizer”
« sensibilisant cutané »
“skin sensitizer” means a mixture or substance that is liable to lead to an allergic response following skin contact.
Classification in a Category or Subcategory of the Class
Classification of Substances
Respiratory sensitizer — category
8.4.1 (1) A substance that is a respiratory sensitizer is classified in the category of this hazard class in accordance with the following table:
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Respiratory Sensitizer — Category 1 | A substance
|
Respiratory sensitizer — subcategories
(2) A substance classified in the category “Respiratory Sensitizer — Category 1” under subsection (1) is, if the applicable data are available, further classified in the subcategory “Respiratory Sensitizer — Category 1A” or in the subcategory “Respiratory Sensitizer — Category 1B” in accordance with the following table:
| Item | Column 1 Category |
Column 2 Subcategory |
Column 3 Criteria |
|---|---|---|---|
| 1. | Respiratory Sensitizer — Category 1 | Respiratory Sensitizer — Category 1A | A substance
|
| 2. | Respiratory Sensitizer — Category 1 | Respiratory Sensitizer — Category 1B | A substance
|
Skin sensitizer — category
(3) A substance that is a skin sensitizer is classified in the category of this hazard class in accordance with the following table:
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Skin Sensitizer — Category 1 | A substance
|
Skin sensitizer — subcategories
(4) A substance classified in the category “Skin Sensitizer — Category 1” under subsection (3) is, if the applicable data are available, further classified in the subcategory “Skin Sensitizer — Category 1A” or in the subcategory “Skin Sensitizer — Category 1B” in accordance with the following table:
| Item | Column 1 Category |
Column 2 Subcategory |
Column 3 Criteria |
|---|---|---|---|
| 1. | Skin Sensitizer — Category 1 | Skin Sensitizer — Category 1A | A substance
|
| 2. | Skin Sensitizer — Category 1 | Skin Sensitizer — Category 1B | A substance
|
Classification of Mixtures
Order of provisions
8.4.2 The classification of a mixture as a respiratory sensitizer or a skin sensitizer, or both, in one or more categories of this hazard class must proceed in accordance with the order of sections 8.4.3 to 8.4.5.
Data available for mixture as a whole
8.4.3 If data of the types referred to in subparagraphs 2.1(a)(i) to (iv) are available for the mixture as a whole, the mixture must be classified as a respiratory sensitizer or a skin sensitizer, or both, in accordance with section 8.4.1.
Data available for use of bridging principles
8.4.4 If data are available to enable the characterization of the mixture as a respiratory sensitizer or a skin sensitizer, or both, in accordance with the bridging principles referred to in subsections 2.3(3) to (8), the mixture must be classified in a category of this hazard class in accordance with those subsections.
Data available for ingredients
8.4.5 A mixture is classified as a respiratory sensitizer or as a skin sensitizer, or both, as the case may be, in accordance with the following:
- (a) as a respiratory sensitizer,
- (i) in the category “Respiratory Sensitizer — Category 1”, if it contains at least one ingredient at a concentration equal to or greater than the concentration limit of 0.1% that is classified in the category “Respiratory Sensitizer — Category 1”,
- (ii) in the subcategory “Respiratory Sensitizer — Category 1A”, if it contains at least one ingredient at a concentration equal to or greater than the concentration limit of 0.1% that is classified in the subcategory “Respiratory Sensitizer — Category 1A”, or
- (iii) in the subcategory “Respiratory Sensitizer — Category 1B”, if it does not contain ingredients classified in the subcategory “Respiratory Sensitizer — Category 1A” at a concentration equal to or greater than the concentration limit of 0.1% and
- (A) it contains at least one ingredient that is a solid or a liquid at a concentration equal to or greater than the concentration limit of 1.0% that is classified in the subcategory “Respiratory Sensitizer — Category 1B”, or
- (B) it contains at least one ingredient that is a gas at a concentration equal to or greater than the concentration limit of 0.2% that is classified in the subcategory “Respiratory Sensitizer — Category 1B”; or
- (b) as a skin sensitizer,
- (i) in the category “Skin Sensitizer — Category 1”, if it contains at least one ingredient at a concentration equal to or greater than the concentration limit of 0.1% that is classified in the category “Skin Sensitizer — Category 1”,
- (ii) in the subcategory “Skin Sensitizer — Category 1A”, if it contains at least one ingredient at a concentration equal to or greater than the concentration limit of 0.1% that is classified in the subcategory “Skin Sensitizer — Category 1A”, or
- (iii) in the subcategory “Skin Sensitizer — Category 1B”, if it does not contain ingredients classified in the subcategory “Skin Sensitizer — Category 1A” at a concentration equal to or greater than the concentration limit of 0.1% and it contains at least one ingredient at a concentration equal to or greater than the concentration limit of 1.0% that is classified in the subcategory “Skin Sensitizer — Category 1B”.
SUBPART 5
GERM CELL MUTAGENICITY
Definitions
Definitions
8.5. The following definitions apply in this Subpart.
“genotoxicity”
« génotoxicité »
“genotoxicity” means the alteration of the structure, information content or segregation of DNA by an agent or process, including those agents or processes that cause DNA damage by interfering with normal replication processes or that in a non-physiological manner temporarily alter its replication.
“germ cell mutagen”
« mutagène des cellules germinales »
“germ cell mutagen” means a mixture or substance that is liable to lead to an increased occurrence of mutations in the germ cells of a population.
“mutagenic”
« mutagène »
“mutagenic” means, in relation to a mixture or substance, liable to lead to an increased occurrence of mutations in populations of cells or organisms.
“mutagenicity”
« mutagénicité »
“mutagenicity” means an increased occurrence of mutations in populations of cells or organisms.
“mutation”
« mutation »
“mutation” means a permanent change in the amount or structure of the genetic material in a cell and includes
- (a) the heritable genetic changes that may be manifested at the phenotypic level; and
- (b) the underlying DNA modifications when known, including specific base pair changes and chromosomal translocations.
Classification in a Category or Subcategory of the Class
Classification of Substances
Categories
8.5.1 A substance that is a germ cell mutagen is classified in a category or subcategory of this hazard class in accordance with the following table:
| Item | Column 1 Category |
Column 2 Subcategory |
Column 3 Criteria |
|---|---|---|---|
| 1. | Germ Cell Mutagenicity — Category 1 | Germ Cell Mutagenicity — Category 1A | A substance that, according to data from human epidemiological studies, induces heritable mutations in germ cells |
| 2. | Germ Cell Mutagenicity — Category 1 | Germ Cell Mutagenicity — Category 1B | A substance in respect of which
|
| 3. | Germ Cell Mutagenicity — Category 2 | A substance in respect of which
|
Classification of Mixtures
Order of provisions
8.5.2 The classification of a mixture as a germ cell mutagen in a category or subcategory of this hazard class must proceed in accordance with the order of sections 8.5.3 to 8.5.5.
Ingredient classified in Category 1A or 1B
8.5.3 A mixture is classified in the category “Germ Cell Mutagenicity — Category 1” if it contains at least one ingredient at a concentration equal to or greater than the concentration limit of 0.1% that is classified in the subcategory “Germ Cell Mutagenicity — Category 1A” or in the subcategory “Germ Cell Mutagenicity — Category 1B”, unless
- (a) there are data for the mixture as a whole that demonstrate conclusively, in accordance with established scientific principles, that the mixture is a germ cell mutagen, in which case the mixture is classified as a germ cell mutagen in accordance with section 8.5.1; or
- (b) the mixture as a whole has been subjected to an in vivo heritable germ cell mutagenicity test that determines that the mixture is not a germ cell mutagen, and a scientifically validated method was used and the test was performed in accordance with generally accepted standards of good scientific practice at the time it was carried out.
Ingredient classified in Category 2
8.5.4 A mixture is classified in the category “Germ Cell Mutagenicity — Category 2” if it contains at least one ingredient at a concentration equal to or greater than the concentration limit of 1.0% that is classified in the category “Germ Cell Mutagenicity — Category 2”, unless
- (a) there are data for the mixture as a whole that demonstrate conclusively, in accordance with established scientific principles, that the mixture is a germ cell mutagen, in which case the mixture is classified as a germ cell mutagen in accordance with section 8.5.1; or
- (b) the mixture as a whole has been subjected to an in vivo heritable germ cell mutagenicity test that determines that the mixture is not a germ cell mutagen, and a scientifically validated method was used and the test was performed in accordance with generally accepted standards of good scientific practice at the time it was carried out.
Data available for use of bridging principles
8.5.5 If data are available to enable the characterization of the mixture as a germ cell mutagen, in accordance with the bridging principles referred to in subsections 2.3(3), (4) and (7), the mixture must be classified in accordance with those subsections.
SUBPART 6
CARCINOGENICITY
Definition
Definition of “carcinogenic”
8.6. In this Subpart, “carcinogenic” means, in relation to a mixture or substance, liable to lead to cancer or increase the incidence of cancer.
Classification in a Category or Subcategory of the Class
Classification of Substances
Categories
8.6.1 A carcinogenic substance is classified in a category or subcategory of this hazard class in accordance with the following table:
| Item | Column 1 Category |
Column 2 Subcategory |
Column 3 Criteria |
|---|---|---|---|
| 1. | Carcinogenicity — Category 1 | Carcinogenicity — Category 1A | A substance in respect of which human data establish a causal relationship between exposure to the substance and the development of cancer |
| 2. | Carcinogenicity — Category 1 | Carcinogenicity — Category 1B | A substance in respect of which
|
| 3. | Carcinogenicity — Category 2 | A substance in respect of which
|
Classification of Mixtures
Order of provisions
8.6.2 The classification of a mixture as a carcinogenic mixture in a category or subcategory of this hazard class must proceed in accordance with the order of sections 8.6.3 to 8.6.5.
Ingredient classified in Category 1A or 1B
8.6.3 A mixture is classified in the category “Carcinogenicity — Category 1” if it contains at least one ingredient at a concentration equal to or greater than the concentration limit of 0.1% that is classified in the subcategory “Carcinogenicity — Category 1A” or in the subcategory “Carcinogenicity — Category 1B”, unless
- (a) there are data for the mixture as a whole that demonstrate conclusively, in accordance with established scientific principles, that the mixture is carcinogenic, in which case the mixture is classified as a carcinogenic mixture in accordance with section 8.6.1; or
- (b) the mixture as a whole has been subjected to a carcinogenicity study that determines that the mixture is not carcinogenic, and a scientifically validated method was used and the study was performed in accordance with generally accepted standards of good scientific practice at the time it was carried out.
Ingredient classified in Category 2
8.6.4 A mixture is classified in the category “Carcinogenicity — Category 2” if it contains at least one ingredient at a concentration equal to or greater than the concentration limit of 0.1% that is classified in the category “Carcinogenicity — Category 2”, unless
- (a) there are data for the mixture as a whole that demonstrate conclusively, in accordance with established scientific principles, that the mixture is carcinogenic, in which case the mixture is classified as a carcinogenic mixture in accordance with section 8.6.1; or
- (b) the mixture as a whole has been subjected to a carcinogenicity study that determines that the mixture is not carcinogenic, and a scientifically validated method was used and the study was performed in accordance with generally accepted standards of good scientific practice at the time it was carried out.
Data available for use of bridging principles
8.6.5 If data are available to enable the characterization of the mixture as carcinogenic, in accordance with the bridging principles referred to in subsections 2.3(3), (4) and (7), the mixture must be classified in accordance with those subsections.
SUBPART 7
REPRODUCTIVE TOXICITY
Definitions
Definitions
8.7. The following definitions apply in this Subpart.
“adverse effects on sexual function and fertility”
« effets néfastes sur la fonction sexuelle et la fertilité »
“adverse effects on sexual function and fertility” means any effect of a mixture or substance that is liable to interfere with sexual function or fertility, including
- (a) alterations to the female or male reproductive system;
- (b) adverse effects on onset of puberty, gamete production or transport, the reproductive cycle, sexual behaviour, parturition or pregnancy outcomes;
- (c) premature reproductive senescence; or
- (d) any modifications to other functions that are dependent on the integrity of the reproductive system.
“adverse effects on the development of the embryo, fetus or offspring”
« effets néfastes sur le développement de l'embryon, du fœtus ou de la progéniture
“adverse effects on the development of the embryo, fetus or offspring” means any adverse effects of a mixture or substance on the embryo, fetus or offspring, resulting from exposure of either parent to the mixture or substance prior to conception or exposure of the developing embryo or fetus to the mixture or substance during prenatal development, or of the offspring during postnatal development to the time of sexual maturation, that is manifested at any point in the development of the embryo or fetus, or that is manifested at any point in the lifespan of the offspring, and that includes the loss of the embryo or fetus, death of the developing offspring, structural abnormality, altered growth and functional deficiency. This definition excludes the induction of genetically based inheritable effects in the offspring.
“effects on or via lactation”
« effets sur ou via l'allaitement »
“effects on or via lactation” means
- (a) any effect of a mixture or substance that interferes with lactation; or
- (b) the presence of the mixture or substance, or its metabolites, in the maternal milk in amounts for which there is evidence that supports the conclusion, in accordance with established scientific principles, that the health of the breast-fed child or suckling animal is liable to be threatened.
“reproductive toxicity”
« toxicité pour la reproduction »
“reproductive toxicity” refers to
- (a) adverse effects on sexual function and fertility;
- (b) adverse effects on the development of the embryo, fetus or offspring; or
- (c) effects on or via lactation.
“toxic to reproduction”
« toxique pour la reproduction »
“toxic to reproduction” means, in relation to a mixture or substance, liable to lead to reproductive toxicity.
Classification in a Category or Subcategory of the Class
Classification of Substances
Categories or subcategories — Categories 1A, 1B and 2
8.7.1 (1) A substance that is toxic to reproduction is classified in a category or subcategory of this hazard class in accordance with the following table:
| Item | Column 1 Category |
Column 2 Subcategory |
Column 3 Criteria |
|---|---|---|---|
| 1. | Reproductive Toxicity — Category 1 | Reproductive Toxicity — Category 1A | A substance in respect of which human data demonstrate that exposure to the substance leads to adverse effects on sexual function and fertility or adverse effects on the development of the embryo, fetus or offspring |
| 2. | Reproductive Toxicity — Category 1 | Reproductive Toxicity — Category 1B | A substance in respect of which animal data demonstrate that exposure of the animal to the substance leads to the following, unless the mechanism or mode of action of the substance in the animal is not relevant to humans:
|
| 3. | Reproductive Toxicity — Category 2 | A substance in respect of which human or animal data support — unless the mechanism or mode of action of the substance in the animal is not relevant to humans — a positive association between exposure to the substance and adverse effects on sexual function and fertility or adverse effects on the development of the embryo, fetus or offspring, but do not support a conclusion, in accordance with established scientific principles, that exposure to the substance leads to such effects |
Category — effects on or via lactation
(2) A substance that is toxic to reproduction is classified in the category of this hazard class in accordance with the following table:
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Reproductive Toxicity — Effects on or via Lactation | A substance that, according to human or animal data, has effects on or via lactation |
Classification of Mixtures
Order of provisions
8.7.2 Subject to subsection 8.7.5(2), the classification of a mixture as a mixture that is toxic to reproduction in a category or subcategory of this hazard class must proceed in accordance with the order of sections 8.7.3 to 8.7.6.
Ingredient classified in Reproductive Toxicity — Category 1A or 1B
8.7.3 A mixture is classified in the category “Reproductive Toxicity — Category 1” if it contains at least one ingredient at a concentration equal to or greater than the concentration limit of 0.1% that is classified in the subcategory “Reproductive Toxicity — Category 1A” or in the subcategory “Reproductive Toxicity — Category 1B”, unless
- (a) there are data for the mixture as a whole that demonstrate conclusively, in accordance with established scientific principles, that the mixture has adverse effects on sexual function and fertility or adverse effects on the development of the embryo, fetus or offspring, in which case the mixture is classified as a mixture that is toxic to reproduction in accordance with subsection 8.7.1(1); or
- (b) the mixture as a whole has been subjected to a reproductive toxicity study that determines that the mixture does not have adverse effects on sexual function and fertility or adverse effects on the development of the embryo, fetus or offspring, and a scientifically validated method was used and the study was performed in accordance with generally accepted standards of good scientific practice at the time it was carried out.
Ingredient classified in Reproductive Toxicity — Category 2
8.7.4 A mixture is classified in the category “Reproductive Toxicity — Category 2” if it contains at least one ingredient at a concentration equal to or greater than the concentration limit of 0.1% that is classified in the category “Reproductive Toxicity — Category 2”, unless
- (a) there are data for the mixture as a whole that demonstrate conclusively, in accordance with established scientific principles, that the mixture has adverse effects on sexual function and fertility or adverse effects on the development of the embryo, fetus or offspring, in which case the mixture is classified as a mixture that is toxic to reproduction in accordance with subsection 8.7.1(1); or
- (b) the mixture as a whole has been subjected to a reproductive toxicity study that determines that the mixture does not have adverse effects on sexual function and fertility or adverse effects on the development of the embryo, fetus or offspring, and a scientifically validated method was used and the study was performed in accordance with generally accepted standards of good scientific practice at the time it was carried out.
Ingredient classified in Reproductive Toxicity — Effects on or via Lactation
8.7.5 (1) A mixture is classified in the category “Reproductive Toxicity — Effects on or via Lactation” if it contains at least one ingredient at a concentration equal to or greater than the concentration limit of 0.1% that is classified in the category “Reproductive Toxicity — Effects on or via Lactation”, unless
- (a) there are data for the mixture as a whole that demonstrate conclusively, in accordance with established scientific principles, that the mixture has effects on or via lactation, in which case the mixture is classified as a mixture that is toxic to reproduction in accordance with subsection 8.7.1(2); or
- (b) the mixture as a whole has been subjected to a reproductive toxicity study that determines that the mixture does not have effects on or via lactation, and a scientifically validated method was used and the study was performed in accordance with generally accepted standards of good scientific practice at the time it was carried out.
Classification in Category 1A, 1B or 2, and in Reproductive Toxicity — Effects on or via Lactation
(2) Despite subsection 2.2(3), a mixture that has been classified in accordance with section 8.7.3 or 8.7.4 and meets the criteria of subsection (1) is also classified in the category “Reproductive Toxicity — Effects on or via Lactation”.
Data available for use of bridging principles
8.7.6 If data are available to enable the characterization of the mixture as toxic to reproduction in accordance with the bridging principles referred to in subsections 2.3(3), (4) and (7), the mixture must be classified in accordance with those subsections, in the following categories:
- (a) “Reproductive Toxicity — Category 1”;
- (b) “Reproductive Toxicity — Category 2”;
- (c) “Reproductive Toxicity — Effects on or via Lactation”;
- (d) both “Reproductive Toxicity — Category 1” and “Reproductive Toxicity — Effects on or via Lactation”; or
- (e) both “Reproductive Toxicity — Category 2” and “Reproductive Toxicity — Effects on or via Lactation”.
SUBPART 8
SPECIFIC TARGET ORGAN TOXICITY — SINGLE EXPOSURE
Definitions
Definitions
8.8. The following definitions apply in this Subpart.
“narcotic effects”
« effets narcotiques »
“narcotic effects” means central nervous system depression that
- (a) in humans, may present as drowsiness, narcosis, reduced alertness, loss of reflexes, lack of coordination, vertigo, severe headache or nausea and may lead to reduced judgment, dizziness, irritability, fatigue, impaired memory function, deficits in perception or coordination, prolonged reaction time or sleepiness; and
- (b) in animals, may be observed as lethargy, lack of coordination righting reflex, narcosis or ataxia.
“organ”
« organe »
“organ” includes any biological system.
“respiratory tract irritation”
« irritation des voies respiratoires »
“respiratory tract irritation” means localized redness, edema, pruritis or irritant effects in the respiratory tract that impair its function, whether or not accompanied by cough, pain, choking, breathing difficulties or other respiratory symptoms.
“specific target organ toxicity arising from a single exposure”
« toxicité pour certains organes cibles à la suite d'une exposition unique »
“specific target organ toxicity arising from a single exposure” means specific, non-lethal toxic effects on target organs that arise from a single exposure to a mixture or substance, including all health effects liable to impair function of the body or any of its parts, whether reversible or irreversible, immediate or delayed, but excludes effects resulting from health hazards addressed by Subparts 1 to 7 and 10 of this Part.
Classification in a Category of the Class
Classification of Substances
Two evaluations
8.8.1 (1) In order to establish the classification of a substance that causes specific target organ toxicity arising from a single exposure in one or more categories of this hazard class, the substance must be evaluated in accordance with all the criteria set out in column 2 of the following table, in relation to toxic effects on
- (a) the central nervous system and respiratory tract; and
- (b) other specific target organs.
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Specific Target Organ Toxicity — Single Exposure — Category 1 | A substance that
|
| 2. | Specific Target Organ Toxicity — Single Exposure — Category 2 | A substance that, according to animal data, causes specific target organ toxicity arising from a single exposure at moderate exposure concentrations, within the concentration value ranges set out for Category 2 in Table 3.8.1 of the GHS, unless the mechanism or mode of action of the substance in the animal is not relevant to humans |
| 3. | Specific Target Organ Toxicity — Single Exposure — Category 3 | A substance in respect of which data demonstrate that a single exposure to the substance generates transient narcotic effects or transient respiratory tract irritation |
Classification
(2) Following the evaluations referred to in subsection (1), the substance is classified in one or more categories of this hazard class, based on the results of the evaluations of its toxic effects as set out in column 1 and column 2 of the following table, in accordance with the corresponding category set out in column 3:
| Item | Column 1 | Column 2 | Column 3 Classification |
|---|---|---|---|
| Item Number of the Table to Subsection (1) that is Associated with the Criteria in Column 2 of that Table that are Determined to Have Been Met as a Result of the Evaluation of the Following: | |||
| Toxic Effects on the Central Nervous System and Respiratory Tract | Toxic Effects on Other Specific Target Organs | Category of the “Specific Target Organ Toxicity — Single Exposure” Hazard Class | |
| 1. | None | Item 1 | Category 1 |
| 2. | Item 1 | None | Category 1 |
| 3. | Item 1 | Item 1 | Category 1 |
| 4. | None | Item 2 | Category 2 |
| 5. | Item 2 | None | Category 2 |
| 6. | Item 1 | Item 2 | Category 1 |
| 7. | Item 2 | Item 1 | Category 1 |
| 8. | Item 2 | Item 2 | Category 2 |
| 9. | Item 3 | None | Category 3 |
| 10. | Item 3 | Item 1 | Category 1 and Category 3 |
| 11. | Item 3 | Item 2 | Category 2 and Category 3 |
Classification of Mixtures
Order of provisions
8.8.2 The classification of a mixture as a mixture that causes specific target organ toxicity arising from a single exposure in a category of this hazard class must proceed in accordance with the order of sections 8.8.3 to 8.8.5.
Data available for mixture as a whole
8.8.3 If data of the types referred to in subparagraphs 2.1(a)(i) to (iv) are available for the mixture as a whole, the mixture must be classified as a mixture that causes specific target organ toxicity arising from a single exposure in accordance with section 8.8.1.
Data available for use of bridging principles
8.8.4 If data are available to enable the characterization of the mixture as a mixture that causes specific target organ toxicity arising from a single exposure, in accordance with the bridging principles referred to in subsections 2.3(3) to (8), the mixture must be classified in one or more categories of this hazard class, based on the table to subsection 8.8.1(2), in accordance with those subsections.
Data available for ingredients — Category 1, 2 or 3
8.8.5 (1) A mixture that contains one or more ingredients that are classified as a substance that causes specific target organ toxicity arising from a single exposure is classified as follows:
- (a) in the category “Specific Target Organ Toxicity — Single Exposure — Category 1”, if it contains at least one ingredient at a concentration equal to or greater than the concentration limit of 1.0% that is classified in the category “Specific Target Organ Toxicity — Single Exposure — Category 1”;
- (b) in the category “Specific Target Organ Toxicity — Single Exposure — Category 2”, if it contains at least one ingredient at a concentration equal to or greater than the concentration limit of 1.0% that is classified in the category “Specific Target Organ Toxicity — Single Exposure — Category 2”; or
- (c) in the category “Specific Target Organ Toxicity — Single Exposure — Category 3”, if it contains at least one ingredient that is classified in the category “Specific Target Organ Toxicity — Single Exposure — Category 3” that is
- (i) at a concentration equal to or greater than the concentration at which the effect is elicited, if known,
- (ii) at a concentration equal to or greater than the concentration limit of 20.0%, or
- (iii) at a concentration that is 1.0% or more that which, when added to the concentration of all other ingredients present individually in a concentration of 1.0% or more and classified in the category “Specific Target Organ Toxicity — Single Exposure — Category 3”, is equal to or greater than the concentration limit of 20.0%.
Data available for ingredients — Category 1 and 3 or Category 2 and 3
(2) Despite subsection 2.2(3), a mixture that has been classified in accordance with paragraph (1)(a) or (b) and meets the criteria of paragraph (1)(c) is also classified in the category “Specific Target Organ Toxicity — Single Exposure — Category 3”.
SUBPART 9
SPECIFIC TARGET ORGAN TOXICITY — REPEATED EXPOSURE
Definitions
Definitions
8.9. The following definitions apply in this Subpart.
“organ”
« organe »
“organ” includes any biological system.
“specific target organ toxicity arising from repeated exposure”
« toxicité pour certains organes cibles à la suite d'expositions répétées »
“specific target organ toxicity arising from repeated exposure” means specific toxic effects on target organs that arise from repeated exposure to a mixture or substance, including all health effects liable to impair function of the body or any of its parts, whether reversible or irreversible, immediate or delayed, but excludes effects resulting from health hazards addressed by Subparts 1 to 7 and 10 of this Part.
Classification in a Category of the Class
Classification of Substances
Categories
8.9.1 A substance that causes specific target organ toxicity arising from repeated exposure is classified in a category of this hazard class in accordance with the following table:
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Specific Target Organ Toxicity — Repeated Exposure — Category 1 | A substance that
|
| 2. | Specific Target Organ Toxicity — Repeated Exposure — Category 2 | A substance that, according to animal data, causes specific target organ toxicity arising from repeated exposure at moderate exposure concentrations, within the concentration value ranges set out in Table 3.9.2 of the GHS, unless the mechanism or mode of action of the substance in the animal is not relevant to humans |
Classification of Mixtures
Order of provisions
8.9.2 The classification of a mixture as a mixture that causes specific target organ toxicity arising from repeated exposure in a category of this hazard class must proceed in accordance with the order of sections 8.9.3 to 8.9.5.
Data available for mixture as a whole
8.9.3 If data of the types referred to in subparagraphs 2.1(a)(i) to (iv) are available for the mixture as a whole, the mixture must be classified as a mixture that causes specific target organ toxicity arising from repeated exposure in accordance with section 8.9.1.
Data available for use of bridging principles
8.9.4 If data are available to enable the characterization of the mixture as a mixture that causes specific target organ toxicity arising from repeated exposure, in accordance with the bridging principles referred to in subsections 2.3(3) to (8), the mixture must be classified in a category of this hazard class in accordance with those subsections.
Data available for ingredients
8.9.5 A mixture that contains one or more ingredients that are classified as a substance that causes specific target organ toxicity arising from repeated exposure is classified as follows:
- (a) in the category “Specific Target Organ Toxicity — Repeated Exposure — Category 1” if it contains at least one ingredient at a concentration equal to or greater than the concentration limit of 1.0% that is classified in the category “Specific Target Organ Toxicity — Repeated Exposure — Category 1”; or
- (b) in the category “Specific Target Organ Toxicity — Repeated Exposure — Category 2” if it contains at least one ingredient at a concentration equal to or greater than the concentration limit of 1.0% that is classified in the category “Specific Target Organ Toxicity — Repeated Exposure — Category 2”.
SUBPART 10
ASPIRATION HAZARD
Definitions
Definitions
8.10. The following definitions apply in this Subpart.
“aspiration toxicant”
« toxique par aspiration »
“aspiration toxicant” means a mixture or substance that is liable to cause aspiration toxicity.
“aspiration toxicity”
« toxicité par aspiration »
“aspiration toxicity” includes severe acute effects, such as chemical pneumonia, varying degrees of pulmonary injury or death, following the entry of a liquid or solid directly through the oral or nasal cavity, or indirectly from vomiting, into the trachea and lower respiratory system.
Classification in the Category of the Class
Classification of Substances
Category
8.10.1 A substance that is an aspiration toxicant is classified in the category of this hazard class in accordance with the following table:
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Aspiration Hazard — Category 1 | A substance that
|
Classification of Mixtures
Order of provisions
8.10.2 The classification of a mixture as an aspiration toxicant in the category of this hazard class must proceed in accordance with the order of sections 8.10.3 to 8.10.5.
Data available for mixture as a whole
8.10.3 If data of the types referred to in subparagraphs 2.1(a)(i) to (iv) are available for the mixture as a whole, the mixture must be classified as an aspiration toxicant in accordance with section 8.10.1.
Data available for use of bridging principles
8.10.4 If data are available to enable the characterization of a mixture as an aspiration toxicant, in accordance with the bridging principles referred to in subsections 2.3(3) to (7), the mixture must be classified in accordance with those subsections. However, subsection 2.3(3) does not apply if the concentration of aspiration toxicant in the mixture is less than the concentration limit of 10.0%.
Data available for ingredients
8.10.5 A mixture that contains one or more ingredients that are classified as an aspiration toxicant is classified in the category “Aspiration Hazard — Category 1” if
- (a) the sum of the concentrations of the ingredients that are present individually at a concentration of 1.0% or more and that are classified in the category “Aspiration Hazard — Category 1” is equal to or greater than the concentration limit of 10.0% and the mixture has a kinematic viscosity less than or equal to 20.5 mm2/s, measured at 40°C; or
- (b) it separates into two or more distinct layers, in one of which the sum of the concentrations of the ingredients that are present individually at a concentration of 1.0% or more and that are classified in the category “Aspiration Hazard — Category 1” is equal to or greater than the concentration limit of 10.0% and the kinematic viscosity of this layer, measured at 40°C, is less than or equal to 20.5 mm2/s.
SUBPART 11
BIOHAZARDOUS INFECTIOUS MATERIALS
Definition
Definition of “biohazardous infectious material”
8.11. In this Subpart, “biohazardous infectious material” means any microorganism, nucleic acid or protein that causes or is a probable cause of infection, with or without toxicity, in humans or animals.
Classification in the Category of the Class
Classification of Substances
Category
8.11.1 A substance that is a biohazardous infectious material is classified in the category of this hazard class in accordance with the following table:
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Biohazardous Infectious Materials — Category 1 | A biohazardous infectious material that
|
Classification of Mixtures
Mixture containing more than one biohazardous infectious material
8.11.2 A mixture that contains one or more ingredients that are classified as a biohazardous infectious material must be classified in accordance with section 8.11.1.
SUBPART 12
HEALTH HAZARDS NOT OTHERWISE CLASSIFIED
Definition
Definition of “health hazard not otherwise classified”
8.12. In this Subpart, “health hazard not otherwise classified” means a health hazard presented by a mixture or substance that is different from any other health hazard addressed by any other Subpart in this Part, that has the characteristic of occurring via acute or repeated exposure and resulting in the death of a person exposed to it or has an adverse effect on that person's health — including an injury.
Classification of Substances
Classification of substances
Category
8.12.1 A substance that presents a health hazard not otherwise classified is classified in the category of this hazard class in accordance with the following table:
| Item | Column 1 Category |
Column 2 Criteria |
|---|---|---|
| 1. | Health Hazards Not Otherwise Classified — Category 1 | A substance that presents a health hazard not otherwise classified |
Classification of Mixtures
Order of provisions
8.12.2 The classification of a mixture as a health hazard not otherwise classified in the category of this hazard class must proceed in accordance with the order of sections 8.12.3 and 8.12.4.
Data available for mixture as a whole
8.12.3 If data of the types referred to in subparagraphs 2.1(a)(i) to (iv) are available for the mixture as a whole, the mixture must be classified as a health hazard not otherwise classified in accordance with section 8.12.1.
Data available for ingredients
8.12.4 A mixture that contains one or more ingredients that are classified as a health hazard not otherwise classified at a concentration equal to or greater than the concentration limit of 1.0% is classified in the category “Health Hazards Not Otherwise Classified — Category 1”.
PART 9
CONSEQUENTIAL AMENDMENTS, TRANSITIONAL PROVISIONS, REPEALS AND COMING INTO FORCE
CONSEQUENTIAL AMENDMENTS
Food and Drug Regulations
9. Subparagraph C.04.413(3)(a)(ii) of the Food and Drug Regulations (see footnote 12) is replaced by the following:
- (ii) the pictogram for biohazardous infectious materials set out in Schedule 3 to the Hazardous Products Regulations; and
Hazardous Materials Information Review Regulations
10. (1) The definition “identificateur du produit” in subsection 2(1) of the French version of the Hazardous Materials Information Review Regulations (see footnote 13) is repealed.
(2) The definitions “original claim” and “refiled claim” in subsection 2(1) of the Regulations are replaced by the following:
“original claim” means a claim for exemption that is filed by a claimant in respect of information relating to a hazardous product, but does not include a refiled claim. (demande originale)
“refiled claim” means a claim for exemption that is filed in respect of information relating to a hazardous product, that is filed by the claimant who filed the original claim relating to that product, and that is solely in respect of any of the information that, under subsection 19(2) of the Act, is or was previously exempt from disclosure in relation to that product. (demande représentée)
(3) The definition “product identifier” in subsection 2(1) of the English version of the Regulations is replaced by the following:
“product identifier” means the chemical name, common name, generic name, trade-name or brand name of a hazardous product. (identificateur de produit)
(4) Subsection 2(1) of the French version of the Regulations is amended by adding the following in alphabetical order:
« identificateur de produit » La marque, la dénomination chimique ou l'appellation courante, commerciale ou générique d'un produit dangereux. (product identifier)
(5) The portion of subsection 2(2) of the Regulations before paragraph (b) is replaced by the following:
(2) For the purposes of the Act, “affected party” means, in respect of a hazardous product that is the subject of a claim for exemption, a person who is not a competitor of the claimant and uses, supplies or is otherwise involved in the use or supply of the hazardous product at a work place, and includes
- (a) a supplier of the hazardous product;
(6) Subsection 2(3) of the Regulations is replaced by the following:
(3) For the purposes of subsection 46(3) of the Act, a medical professional is a nurse who is registered or licensed under the laws of a province to practise nursing and who is practising nursing under those laws in that province.
11. Paragraphs 8(1)(e) to (g) of the Regulations are replaced by the following:
- (e) if the claim is made by a supplier, a statement identifying the subject matter of the information for which the claim is made as being one or more of the following:
- (i) in the case of a material or substance that is a hazardous product,
- (A) the chemical name of the material or substance,
- (B) the CAS registry number, or any other unique identifier, of the material or substance, or
- (C) the chemical name of any impurity, stabilizing solvent or stabilizing additive in the material or substance that is known to the supplier that, in accordance with the provisions of the Hazardous Products Act, is classified in a category or subcategory of a health hazard class and that contributes to the classification, in accordance with those provisions, of the material or substance in the health hazard class,
- (ii) in the case of an ingredient in a mixture that is a hazardous product,
- (A) the chemical name of the ingredient,
- (B) the CAS registry number, or any other unique identifier, of the ingredient, or
- (C) the concentration or concentration range of the ingredient, and
- (iii) in the case of a material, substance or mixture that is a hazardous product, the name of any toxicological study that identifies the material or substance or any ingredient in the mixture;
- (i) in the case of a material or substance that is a hazardous product,
- (f) if the claim is made by an employer, a statement identifying the subject matter of the information for which the claim is made as being one or more of the following:
- (i) in the case of a material or substance that is a hazardous product,
- (A) the chemical name of the material or substance,
- (B) the CAS registry number, or any other unique identifier, of the material or substance, or
- (C) the chemical name of any impurity, stabilizing solvent or stabilizing additive in the material or substance that is known to the employer that, in accordance with the provisions of the Hazardous Products Act, is classified in a category or subcategory of a health hazard class and that contributes to the classification, in accordance with those provisions, of the material or substance in the health hazard class,
- (ii) in the case of an ingredient in a mixture that is a hazardous product,
- (A) the chemical name of the ingredient,
- (B) the CAS registry number, or any other unique identifier, of the ingredient, or
- (C) the concentration or concentration range of the ingredient,
- (iii) in the case of a material, substance or mixture that is a hazardous product, the name of any toxicological study that identifies the material or substance or any ingredient in the mixture,
- (iv) the product identifier of a hazardous product,
- (v) information in respect of a hazardous product, other than the product identifier, that constitutes a means of identification, and
- (vi) information that could be used to identify a supplier of a hazardous product; and
- (i) in the case of a material or substance that is a hazardous product,
- (g) the following in respect of the hazardous product that is the subject of the claim:
- (i) its product identifier,
- (ii) if the claim is a refiled claim, the registry number of the preceding claim filed in respect of that hazardous product,
- (iii) if the claim relates to a material or substance, or an ingredient, an impurity, a stabilizing additive or a stabilizing solvent of the hazardous product, the generic chemical name of the material, substance, ingredient, impurity, stabilizing additive or stabilizing solvent, and
- (iv) the chemical name and, if any, the CAS registry number and any other unique identifier of all materials, substances, ingredients, impurities, stabilizing additives and stabilizing solvents in the hazardous product and their concentrations.
12. Paragraph 10(b) of the Regulations is replaced by the following:
- (b) the safety data sheet or label to which the claim for exemption relates; and
13. Paragraph 11.2(b) of the Regulations is replaced by the following:
- (b) the product identifier of the hazardous product that is the subject of the claim for exemption;
Hazardous Materials Information Review Act Appeal Board Procedures Regulations
14. (1) Paragraph 38(1)(b) of the Hazardous Materials Information Review Act Appeal Board Procedures Regulations (see footnote 14) is replaced by the following:
- (b) the product identifier of the hazardous product that is the subject of the claim for exemption that is the subject of the appeal;
(2) Subsection 38(2) of the French version of the Regulations is replaced by the following:
(2) Pour l'application du paragraphe (1), « identificateur de produit » s'entend au sens du paragraphe 2(1) du Règlement sur le contrôle des renseignements relatifs aux matières dangereuses.
15. (1) Paragraph (c) of Part III of Form 1 of the schedule to the Regulations is replaced by the following:
- (c) a claimant appealing an order made under section 17 of the Act in respect of the compliance of a safety data sheet or label with the provisions of the Hazardous Products Act or the Canada Labour Code, as the case may be
(2) Paragraph (g) of Part III of Form 1 of the schedule to the Regulations is replaced by the following:
- (g) an affected party appealing an order made under section 17 of the Act in respect of the compliance of a safety data sheet or label with the provisions of the Hazardous Products Act or the Canada Labour Code, as the case may be
16. The Regulations are amended by replacing “controlled product” with “hazardous product” in the following provisions:
- (a) Part II of Form 1 of the schedule;
- (b) Part II of Form 2 of the schedule; and
- (c) Form 4 of the schedule.
Consumer Chemicals and Containers Regulations, 2001
17. Paragraph 30(b) of the Consumer Chemicals and Containers Regulations, 2001 (see footnote 15) is replaced by the following:
- (b) must be different from any other border on the label; and
Safety of Human Cells, Tissues and Organs for Transplantation Regulations
18. The Safety of Human Cells, Tissues and Organs for Transplantation Regulations (see footnote 16) are amended by replacing “The hazard symbol entitled “Biohazardous Infectious Material” set out in Schedule II to the Controlled Products Regulations, if applicable” with “The pictogram entitled “Biohazardous Infectious Materials” set out in Schedule 3 to the Hazardous Products Regulations, if applicable” in the following provisions:
- (a) the portion of item 7 of the table to subsection 30(1) in column 1;
- (b) the portion of item 7 of the table to subsection 30(2) in column 1;
- (c) the portion of item 6 of the table to section 31 in column 1; and
- (d) the portion of item 6 of the table to section 32 in column 1.
TRANSITIONAL PROVISIONS
Definitions
19. (1) The following definitions apply in this section.
“controlled product”
« produit contrôlé »
“controlled product” has the same meaning as in section 2 of the Hazardous Products Act as it read immediately before the day on which subsection 111(1) of the Economic Action Plan 2014 Act, No. 1 comes into force.
“former Regulations”
« règlements antérieurs »
“former Regulations” means the Controlled Products Regulations and the Ingredient Disclosure List as they each read immediately before the day on which these Regulations come into force.
Compliance — supplier
(2) These Regulations do not apply to a supplier in respect of the sale or importation of a controlled product that is a hazardous product, as defined in section 2 of the Hazardous Products Act as enacted by subsection 111(3) of the Economic Action Plan 2014 Act, No. 1, if the supplier sells or imports the controlled product on or after the first day on which sections 114 and 115 of the Economic Action Plan 2014 Act, No. 1 are in force, but before a day to be fixed by order of the Governor in Council for the purposes of subsections 130(1) and (2), section 133 and subsection 135(1) of the Economic Action Plan 2014 Act, No. 1, and if the supplier would not be in contravention of the former Regulations were they in force at the time.
Hazardous product that is not controlled product
(3) These Regulations do not apply to a supplier in respect of the sale or importation of a hazardous product, as defined in section 2 of the Hazardous Products Act as enacted by subsection 111(3) of the Economic Action Plan 2014 Act, No. 1 that is not a controlled product, if the supplier sells or imports the hazardous product on or after the first day on which sections 114 and 115 of the Economic Action Plan 2014 Act, No. 1 are in force, but before a day to be fixed by order of the Governor in Council for the purposes of subsection 130(3) of the Economic Action Plan 2014 Act, No. 1.
Compliance — supplier
(4) These Regulations do not apply to a supplier to whom a controlled product that is a hazardous product, as defined in section 2 of the Hazardous Products Act as enacted by subsection 111(3) of the Economic Action Plan 2014 Act, No. 1 is sold, if the supplier sells the controlled product on or after the first day on which sections 114 and 115 of the Economic Action Plan 2014 Act, No. 1 are in force, but before a day to be fixed by order of the Governor in Council for the purposes of subsections 131(1), 134(1) and 136(1) of the Economic Action Plan 2014 Act, No. 1, and if the supplier would not be in contravention of the former Regulations were they in force at the time.
Hazardous product that is not controlled product
(5) These Regulations do not apply to a supplier to whom a hazardous product, as defined in section 2 of the Hazardous Products Act as enacted by subsection 111(3) of the Economic Action Plan 2014 Act, No. 1 that is not a controlled product, has been sold if the supplier sells the hazardous product on or after the first day on which sections 114 and 115 of the Economic Action Plan 2014 Act, No. 1 are in force, but before a day to be fixed by order of the Governor in Council for the purposes of subsection 131(2) of the Economic Action Plan 2014 Act, No. 1.
Compliance — importation — own use in work place
(6) These Regulations do not apply to a supplier in respect of the importation of a controlled product that is a hazardous product, as defined in section 2 of the Hazardous Products Act as enacted by subsection 111(3) of the Economic Action Plan 2014 Act, No. 1, if the supplier imports the controlled product only for use in their work place on or after the first day on which sections 114 and 115 of the Economic Action Plan 2014 Act, No. 1 are in force, but before a day to be fixed by order of the Governor in Council for the purposes of subsections 132(1), 134(2) and 137(1) of the Economic Action Plan 2014 Act, No. 1, and if the supplier would not be in contravention of the former Regulations were they in force at the time.
Hazardous product that is not controlled product
(7) These Regulations do not apply to a supplier in respect of the importation of a hazardous product, as defined in section 2 of the Hazardous Products Act as enacted by subsection 111(3) of the Economic Action Plan 2014 Act, No. 1 that is not a controlled product, if the supplier imports the hazardous product only for use in their work place, on or after the first day on which sections 114 and 115 of the Economic Action Plan 2014 Act, No. 1 are in force, but before a day to be fixed by order of the Governor in Council for the purposes of subsection 132(2) of the Economic Action Plan 2014 Act, No. 1.
REPEALS
20. The Ingredient Disclosure List (see footnote 17) is repealed.
21. The Controlled Products Regulations (see footnote 18) are repealed.
COMING INTO FORCE
S.C. 2014, c. 20
22. These Regulations come into force on the first day on which sections 114 and 115 of the Economic Action Plan 2014 Act, No. 1 come into force.
SCHEDULE 1
(Paragraphs 4(1)(a) and (b), subsections 4(2) and (3), section 4.1 and subsections 5(6), 5.6(2) and (3), 5.7(5) to (10), 5.8(1) and 5.9(1))
| Item | Column 1 Heading |
Column 2 Specific Information Elements |
|---|---|---|
| 1. | Identification |
|
| 2. | Hazard identification |
|
| 3. | Composition/Information on ingredients | (1) In the case of a hazardous product that is a material or substance,
|
| 4. | First aid measures |
|
| 5. | Firefighting measures |
|
| 6. | Accidental release measures |
|
| 7. | Handling and storage |
|
| 8. | Exposure controls/Personal protection |
|
| 9. | Physical and chemical properties |
|
| 10. | Stability and reactivity |
|
| 11. | Toxicological information | Concise but complete description of the various toxic health effects and the data used to identify those effects, including
|
| 12. | Ecological information |
|
| 13. | Disposal considerations | Information on safe handling for disposal and methods of disposal, including any contaminated packaging |
| 14. | Transport information |
|
| 15. | Regulatory information | Safety, health and environmental regulations, made within or outside Canada, specific to the product in question |
| 16. | Other information | Date of the latest revision of the safety data sheet |
SCHEDULE 2
(Subsection 4(4))
| Item | Column 1 Heading |
Column 2 Specific Information Elements |
|---|---|---|
| 1. | Section I — Infectious Agent |
|
| 2. | Section II — Hazard Identification |
|
| 3. | Section III — Dissemination |
|
| 4. | Section IV — Stability and Viability |
|
| 5. | Section V — First Aid/Medical |
|
| 6. | Section VI — Laboratory Hazard |
|
| 7. | Section VII — Exposure Controls/Personal Protection |
|
| 8. | Section VIII — Handling and Storage |
|
| 9. | Section IX — Regulatory and Other Information |
|
SCHEDULE 3
(Subsection 3(3), section 3.1, paragraph 5.3(c) and Schedule 5)
| Item | Column 1 Name of Symbol |
Column 2 Symbol |
Column 3 Pictogram |
|---|---|---|---|
| 1. | Flame | |
|
| 2. | Flame over circle | |
|
| 3. | Exploding bomb | |
|
| 4. | Corrosion | |
|
| 5. | Gas cylinder | |
|
| 6. | Skull and crossbones | |
|
| 7. | Exclamation mark | |
|
| 8. | Health hazard | |
|
| 9. | Biohazardous infectious materials | |
|
SCHEDULE 4
(Subsections 2(3) to (5))
| Item | Column 1 Chemical Name/Description |
Column 2 UN Number or CAS Registry Number |
Column 3 Classification |
|---|---|---|---|
| 1. | Ammonium picrate, wetted with not less than 10.0% water, by mass | 1310 | Physical Hazards Not Otherwise Classified — Category 1 |
| 2. | Dinitrophenol, wetted with not less than 15.0% water, by mass | 1320 | Physical Hazards Not Otherwise Classified — Category 1 |
| 3. | Dinitrophenolates, wetted with not less than 15.0% water, by mass | 1321 | Physical Hazards Not Otherwise Classified — Category 1 |
| 4. | Dinitroresorcinol, wetted with not less than 15.0% water, by mass | 1322 | Physical Hazards Not Otherwise Classified — Category 1 |
| 5. | Nitroguanidine or picrite, wetted with not less than 20.0% water, by mass | 1336 | Physical Hazards Not Otherwise Classified — Category 1 |
| 6. | Nitrostarch, wetted with not less than 20.0% water, by mass | 1337 | Physical Hazards Not Otherwise Classified — Category 1 |
| 7. | Trinitrophenol, wetted with not less than 30.0% water, by mass | 1344 | Physical Hazards Not Otherwise Classified — Category 1 |
| 8. | Silver picrate, wetted with not less than 30.0% water, by mass | 1347 | Physical Hazards Not Otherwise Classified — Category 1 |
| 9. | Sodium dinitro-o-cresolate, wetted with not less than 15.0% water, by mass | 1348 | Physical Hazards Not Otherwise Classified — Category 1 |
| 10. | Sodium picramate, wetted with not less than 20.0% water, by mass | 1349 | Physical Hazards Not Otherwise Classified — Category 1 |
| 11. | Trinitrobenzene, wetted with not less than 30.0% water, by mass | 1354 | Physical Hazards Not Otherwise Classified — Category 1 |
| 12. | Trinitrobenzoic acid, wetted with not less than 30.0% water, by mass | 1355 | Physical Hazards Not Otherwise Classified — Category 1 |
| 13. | Trinitrotoluene, wetted with not less than 30.0% water, by mass | 1356 | Physical Hazards Not Otherwise Classified — Category 1 |
| 14. | Urea nitrate, wetted with not less than 20.0% water, by mass | 1357 | Physical Hazards Not Otherwise Classified — Category 1 |
| 15. | Zirconium picramate, wetted with not less than 20.0% water, by mass | 1517 | Physical Hazards Not Otherwise Classified — Category 1 |
| 16. | Barium azide, wetted with not less than 50.0% water, by mass | 1571 | Physical Hazards Not Otherwise Classified — Category 1 |
| 17. | Nitrocellulose with water (not less than 25.0% water, by mass) | 2555 | Physical Hazards Not Otherwise Classified — Category 1 |
| 18. | Nitrocellulose with alcohol (not less than 25.0% alcohol, by mass, and not more than 12.6% nitrogen, by dry mass) | 2556 | Physical Hazards Not Otherwise Classified — Category 1 |
| 19. | Nitrocellulose mixture, with not more than 12.6% nitrogen, by dry mass, with or without plasticizer, with or without pigment | 2557 | Physical Hazards Not Otherwise Classified — Category 1 |
| 20. | Dipicryl sulfide, wetted with not less than 10.0% water, by mass | 2852 | Physical Hazards Not Otherwise Classified — Category 1 |
| 21. | Isosorbide dinitrate mixture with not less than 60.0% lactose, mannose, starch or calcium hydrogen phosphate | 2907 | Physical Hazards Not Otherwise Classified — Category 1 |
| 22. | Nitrocellulose membrane filters, with not more than 12.6% nitrogen, by dry mass | 3270 | Physical Hazards Not Otherwise Classified — Category 1 |
| 23. | 5-Tert-butyl-2,4,6-trinitrom-xylene or Musk xylene | 2956 | Physical Hazards Not Otherwise Classified — Category 1 |
| 24. | 2-Bromo-2-nitropropane1,3-diol | 3241 | Physical Hazards Not Otherwise Classified — Category 1 |
| 25. | Isosorbide-5-mononitrate with less than 30.0% non-volatile, non-flammable phlegmatizer | 3251 | Physical Hazards Not Otherwise Classified — Category 1 |
| 26. | Azodicarbonamide, technically pure or preparations having a SADT higher than 75°C | 3242 | Physical Hazards Not Otherwise Classified — Category 1 |
| 27. | Nitroglycerin mixture, desensitized, solid, n.o.s., with more than 2.0% but not more than 10.0% nitroglycerin, by mass | 3319 | Physical Hazards Not Otherwise Classified — Category 1 |
| 28. | Pentaerythritol tetranitrate mixture, desensitized, solid, n.o.s., with more than 10.0% but not more than 20.0% pentaerythrite tetranitrate (PETN) by mass | 3344 | Physical Hazards Not Otherwise Classified — Category 1 |
| 29. | Chlorine dioxide | CAS 10049-04-4 | Physical Hazards Not Otherwise Classified — Category 1 |
| 30. | Chloropicrin | 1580 | Physical Hazards Not Otherwise Classified — Category 1 |
| 31. | Nitromethane | 1261 | Physical Hazards Not Otherwise Classified — Category 1 |
| 32. | Ozone | CAS 10028-15-6 | Physical Hazards Not Otherwise Classified — Category 1 |
| 33. | Perchloric acid solutions > 72.0% | CAS 7601-90-3 | Physical Hazards Not Otherwise Classified — Category 1 |
| 34. | Self-heating liquid, organic, n.o.s. | 3183 | Self-heating Substances and Mixtures — Category 1 |
| 35. | Self-heating liquid, toxic, organic, n.o.s. | 3184 | Self-heating Substances and Mixtures — Category 1 |
| 36. | Self-heating liquid, corrosive, organic, n.o.s. | 3185 | Self-heating Substances and Mixtures — Category 1 |
| 37. | Self-heating liquid, inorganic, n.o.s. | 3186 | Self-heating Substances and Mixtures — Category 1 |
| 38. | Self-heating liquid, toxic, inorganic, n.o.s. | 3187 | Self-heating Substances and Mixtures — Category 1 |
| 39. | Self-heating liquid, corrosive, inorganic, n.o.s. | 3188 | Self-heating Substances and Mixtures — Category 1 |
SCHEDULE 5
(Subsection 1(1), subparagraph 3(1)(d)(i) and subsection 3(3))
INFORMATION ELEMENTS FOR SPECIFIED CATEGORIES
PART 1
| Item | Column 1 Category |
Column 2 Name of Symbol |
Column 3 Symbol |
Column 4 Signal Word |
Column 5 Hazard Statement |
|---|---|---|---|---|---|
| 1. | Combustible Dusts — Category 1 | No symbol | No symbol | Warning | May form combustible dust concentrations in air |
PART 2
| Item | Column 1 Category |
Column 2 Name of Symbol |
Column 3 Symbol |
Column 4 Signal Word |
Column 5 Hazard Statement |
|---|---|---|---|---|---|
| 1. | Simple Asphyxiants — Category 1 | No symbol | No symbol | Warning | May displace oxygen and cause rapid suffocation |
PART 3
| Item | Column 1 Category |
Column 2 Name of Symbol |
Column 3 Symbol |
Column 4 Signal Word |
Column 5 Hazard Statement |
|---|---|---|---|---|---|
| 1. | Pyrophoric Gases — Category 1 | Flame | |
Danger | Catches fire spontaneously if exposed to air |
PART 4
| Item | Column 1 Category |
Column 2 Name of Symbol |
Column 3 Symbol |
Column 4 Signal Word |
Column 5 Hazard Statement |
|---|---|---|---|---|---|
| 1. | Physical Hazards Not Otherwise Classified — Category 1 | (Name of any symbol in Schedule 3 that is applicable to the hazard) | (Any symbol in Schedule 3 that is applicable to the hazard) | Danger | (Wording that describes the nature of the hazard) |
PART 5
| Item | Column 1 Category |
Column 2 Name of Symbol |
Column 3 Symbol |
Column 4 Signal Word |
Column 5 Hazard Statement |
|---|---|---|---|---|---|
| 1. | Biohazardous Infectious Materials — Category 1 | Biohazardous infectious materials | |
Danger | (Wording that describes the nature of the hazard) |
PART 6
| Item | Column 1 Category |
Column 2 Name of Symbol |
Column 3 Symbol |
Column 4 Signal Word |
Column 5 Hazard Statement |
|---|---|---|---|---|---|
| 1. | Health Hazards Not Otherwise Classified — Category 1 | (Name of any symbol in Schedule 3 that is applicable to the hazard) | (Any symbol in Schedule 3 that is applicable to the hazard) | Danger | (Wording that describes the nature of the hazard) |
[32-1-o]